OSHA BLOODBORNE PATHOGENS STANDARD
[Content in this section of the course is taken from the OSHA Bloodborne Pathogens Standard (OSHA, 2019).]
The Occupational Safety and Health Administration, part of the U.S. Department of Labor, first published the Occupational Exposure to Bloodborne Pathogens Standard in 1991 in Title 29 of the Code of Federal Regulations 1910.1030. In 2001, in response to the Needlestick Safety and Prevention Act, OSHA revised the Bloodborne Pathogens Standard.
The Bloodborne Pathogens Standard continues to be updated regularly, with the most recent update from May 2019 (see “Resources” at the end of this course). The Standard details what employers must do to protect workers whose jobs put them at risk for exposure to blood and other potentially infectious materials (OPIM). OSHA regularly inspects healthcare agencies for compliance and may fine employers if infractions are identified.
In general, OSHA’s Bloodborne Pathogens Standard requires employers to:
- Provide information and training to employees that covers all elements of the Standard, including, but not limited to, information on bloodborne pathogens and diseases, methods used to control occupational exposure, hepatitis B vaccine, and medical evaluation and postexposure follow-up procedures
- Offer this training on initial assignment, at least annually thereafter, and when new or modified tasks or procedures affect a worker’s occupational exposure
Following is a description of specific elements of the Standard, but this list is not comprehensive. (See “Resources” below for a link to the full Standard.)
BLOOD AND OTHER POTENTIALLY INFECTIOUS MATERIALS
All occupational exposures to blood or other potentially infectious materials place workers at risk for infection with bloodborne pathogens.
OSHA defines blood as:
- Human blood
- Human blood components
- Products made from human blood
Other potentially infectious materials (OPIM) include:
- Semen
- Vaginal secretions
- Cerebrospinal fluid
- Synovial fluid
- Pleural fluid
- Pericardial fluid
- Peritoneal fluid
- Amniotic fluid
- Saliva in dental procedures
- Any body fluid that is visibly contaminated with blood
- All body fluids in situations where it is difficult or impossible to differentiate between them
- Any unfixed tissue or organ (other than intact skin) from a human (living or dead)
- HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions
- Blood, organs, or other tissues from experimental animals infected with HBV or HIV
Occupational exposure to human breast milk has not been shown to lead to transmission of bloodborne pathogens. However, it has been implicated in transmitting HIV from mother to infant. Therefore, gloves may be worn as a precaution by healthcare workers who are frequently exposed to breast milk.
(OSHA, 2019; CDC, 2022a)
Exposure Control Plan
Each employer having employees with occupational exposure shall establish a written exposure control plan outlining processes and procedures to prevent and correct exposure of potential infectious diseases and to provide employee training.
- Prepare an exposure determination that includes the names, department, and task of each employee where the potential for occupational exposure to body fluids exists.
- Ensure a copy of the exposure control plan is accessible to employees.
- Review and update the exposure control plan at least annually and whenever necessary to reflect new or modified tasks and procedures that affect occupational exposure and to reflect new or revised employee positions with occupation exposure. The review and update shall also:
- Reflect changes in technology that eliminate or reduce exposure to bloodborne pathogens
- Document annually consideration and implementation of appropriate commercially available and effective safer medical devices
- Document that they have solicited input from frontline workers in identifying, evaluating, and selecting effective engineering and work practice controls
Universal Precautions
- Universal precautions shall be observed to prevent contact with blood or other potentially infectious materials. Under circumstances in which differentiation between body fluid types is difficult or impossible, all body fluids shall be considered potentially infectious materials.
Engineering and Work Practice Controls
- Engineering and work practice controls shall be used to eliminate or minimize employee exposure. Where exposure remains after institution of these controls, personal protective equipment shall also be used. (See also “Personal Protective Equipment” below.)
- Engineering controls shall be examined and maintained or replaced on a regular schedule to ensure their effectiveness.
ENGINEERING CONTROL DEVICE EXAMPLES
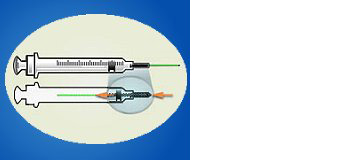
Retractable needle.
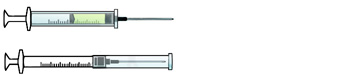
Self-resheathing needle.

Resheathing disposable scalpel.

Phlebotomy needle with hinged shield as an add-on safety feature.
(OSHA, 2020)
- Employers shall provide handwashing facilities that are readily accessible to employees.
- When provision of handwashing facilities is not feasible, the employer shall provide either an appropriate antiseptic hand cleanser in conjunction with a clean cloth or paper towels or antiseptic towelettes. When antiseptic hand cleansers or towelettes are used, hands shall be washed with soap and running water as soon as feasible.
- Employers should ensure that employees wash their hands immediately or as soon as feasible after removal of gloves or other personal protective equipment.
- Employers shall ensure employees wash hands and skin with soap and water or flush mucous membranes with water immediately or as soon as feasible following contact with blood or OPIM. (See also “Hand Hygiene” later in this course.)
- Contaminated needles and other contaminated sharps shall not be bent, recapped, or removed unless the employer can demonstrate that no alternative is feasible or that such action is required by a specific medical or dental procedure. Such bending, recapping, or needle removal must be accomplished through the use of a mechanical device or a one-handed technique. Shearing or breaking of contaminated needles is prohibited. (See also “Sharps Handling” later in this course.)
- Immediately or as soon as possible after use, contaminated reusable sharps shall be placed in appropriate containers until properly reprocessed.
- Employers must prohibit eating, drinking, smoking, applying cosmetics or lip balm, and handling contact lenses in work areas where there is a reasonable likelihood of occupational exposure.
- Food and drink shall not be kept in refrigerators, freezers, shelves, cabinets, or on countertops or benchtops where blood or OPIM are present.
- All procedures involving blood or OPIM shall be performed in such a manner as to minimize splashing, spraying, spattering, and generation of droplets of these substances.
- Mouth piping or suctioning of blood or OPIM is prohibited.
- Specimens of blood or OPIM shall be placed in a container that prevents leakage during collection, handling, processing, storage, transport, or shipping.
- Equipment that may become contaminated with blood or other potentially infectious materials shall be examined prior to servicing or shipping and shall be decontaminated as necessary, unless the employer can demonstrate that decontamination of such equipment or portions of such equipment is not feasible.
Personal Protective Equipment
- Employers shall provide at no cost to the employee personal protective equipment (PPE), such as, but not limited to, gloves, gowns, laboratory coats, face shields or masks and eye protection, mouthpieces, resuscitation bags, pocket masks, or other ventilation devices.
- Employers shall ensure that appropriate personal protective equipment in the appropriate sizes is readily accessible at the worksite or is issued to employees. Hypoallergenic gloves, glove liners, powderless gloves, or other similar alternatives shall be readily accessible to those who are allergic to the gloves normally provided.
- Employers must clean, repair, and replace this equipment as needed. Provision, maintenance, repair, and replacement are at no cost to the employee.
- Garments penetrated by blood or other potentially infectious materials shall be removed immediately or as soon as feasible.
- All personal protective equipment shall be removed prior to leaving the work area; when personal protective equipment is removed it shall be placed in an appropriately designated area or container for storage, washing, decontamination, or disposal.
(See also “Standard Precautions, Personal Protective Equipment” later in this course.)
Housekeeping and Regulated Waste Disposal
- Employers shall ensure the worksite is maintained in a clean and sanitary condition. The employer shall determine and implement an appropriate written schedule for cleaning and method of decontamination based upon the location within the facility, type of surface to be cleaned, type of soil present, and tasks or procedures being performed in the area.
- Contaminated sharps shall be discarded immediately or as soon as feasible in containers that are closeable, puncture resistant, leakproof, and labeled or color-coded.
- Employers must ensure that employees who have contact with contaminated laundry wear protective gloves and other appropriate personal protective equipment.
HIV, HBV, and Vaccination and Postexposure Requirements
- HIV and HBV laboratory and production facility workers must receive specialized initial training in addition to the training provided to all workers with occupational exposure risks.
- Hepatitis B vaccination must be offered after the worker has received the required bloodborne pathogens training and within 10 days of initial assignment to a job with occupational exposure.
- Employers shall make available hepatitis B vaccinations to all workers with occupational exposure and post-exposure evaluation and follow-up to all employees who have had an exposure incident. (See also “Postexposure Measures and Follow-Up” later in this course.)
- The healthcare professional will provide a limited written opinion to the employer, and all diagnoses must remain confidential.