ENGINEERING AND WORK PRACTICE CONTROLS
ELEMENT III
Use of engineering and work practice controls to reduce the opportunity for patient and healthcare worker exposure to potentially infectious material in all healthcare settings
In addition to the precautions described above, other practices and controls can be employed to prevent and control infection. These include:
- Engineering controls
- Work practice controls
- Environmental controls
Types of Practices and Controls
Engineering controls are measures that protect workers by removing hazardous conditions or by placing a barrier between the worker and the hazard. Examples include:
- Sharps disposal containers
- Self-sheathing needles
- Sharps with engineered sharps injury protections
- Needleless systems
According to the Occupational Safety and Health Administration (OSHA, 2020b), engineering controls shall be examined and maintained or replaced on a regular schedule to ensure their effectiveness.
ENGINEERING CONTROL DEVICE EXAMPLES
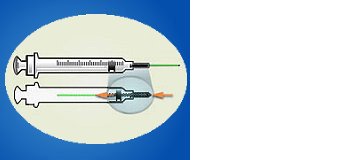
Retractable needle.
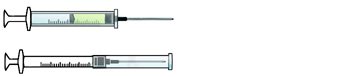
Self-resheathing needle.

Resheathing disposable scalpel.

Phlebotomy needle with hinged shield as an add-on safety feature.
(OSHA, 2020)
Work practice controls reduce the likelihood of exposure to pathogens by changing the way a task is performed, such as:
- Practices for handling and disposing of contaminated sharps
- Handling specimens
- Handling laundry
- Cleaning contaminated surfaces and items
- Performing hand hygiene
(CDC, 2023c; OSHA, 2023a)
Environmental controls help prevent the transmission of infection by reducing the concentration of pathogens in the environment. Such measures include but are not limited to:
- Appropriate ventilation (including air filtration systems)
- Environmental cleaning (housekeeping)
- Cleaning and disinfecting strategies (including food service areas)
- Cleaning, disinfection, and sterilization of patient-care equipment
- Proper linen and laundry management
- Disposal of regulated medical waste
(CDC, 2023c)
Sharps- and Injection-Related Practices and Controls
Engineering, work practice, and environmental controls have all been developed to prevent and control the spread of infection related to the use of needles and other sharps in the healthcare setting.
SHARPS HANDLING
OSHA requirements for handling sharps state that contaminated sharps are needles, blades (such as scalpels), scissors, and other medical instruments and objects that can puncture the skin. Contaminated sharps must be properly disposed of immediately or as soon as possible in containers that are closable, puncture resistant, leakproof on the sides and bottom, and color-coded or labeled with a biohazard symbol.
- Discard needle/syringe units without attempting to recap the needle whenever possible.
- If a needle must be recapped, never use both hands. Use the single-hand “scoop” method by placing the cap on a horizontal surface, gently sliding the needle into the cap with the same hand, tipping the needle up to allow the cap to slide down over the needle, and securing the cap over the needle with the same hand.
- Never break or shear needles.
- To move or pick up needles, use a mechanical device or tool, such as forceps, pliers, or broom and dustpan.
- Use blunt-tip suture needles to decrease risk of percutaneous injury.
- Use only sharps with a safety mechanism attached, and activate the safety mechanism as soon as the device has been used.
- Use luer-lock syringes whenever possible to promote a secure connection between the needle and the syringe.
- Substitute plastic material for glass wherever possible.
- Dispose of needles in labeled sharps containers only. Sharps containers must be accessible and maintained upright.
- When transporting sharps containers, close the containers immediately before removal or replacement to prevent spillage or protrusion of contents during handling or transport.
- Fill a sharps container up to the fill line or two thirds full. Do not overfill the container.
(CU, 2023)
SAFE INJECTION PRACTICES
The following recommendations apply to the use of needles, cannulas that replace needles, and, where applicable, intravenous delivery systems.
- Draw up medications in a designated clean medication preparation area that is not adjacent to potential sources of contamination, including sinks or other water sources.
- Perform hand hygiene prior to preparing medications.
- Access parenteral medications in an aseptic manner, using a new sterile syringe and sterile needle.
- Prepare an injection as close as possible to the time of administration to prevent compromised sterility.
- Always enter a medication vial with a sterile needle and sterile syringe, even when obtaining additional doses of medication for the same patient, and discard after use.
- Use a syringe and needle only once to administer a medication to a single patient.
- Discard medications according to the manufacturer’s expiration date (even if not opened) and whenever sterility is compromised or questionable.
- If a multidose vial has been opened or accessed, the vial should be dated and discarded within 28 days unless manufacturer specifies a different length of time or date.
- Single-dose vials that have been opened or accessed should be discarded according to the manufacturer’s time specifications or at the end of the case/procedure for which they are being used. Do not store for future use.
(OSHA, 2020, 2023a)