THE EXPERIENCE OF PAIN
Pain is the single most common reason people seek medical care (NCCIH, 2022). It is a complex experience that includes a physiologic and a psychological response to noxious stimuli. It is a warning mechanism protecting an individual by influencing them to withdraw from harmful stimuli and is primarily associated with injury or the threat of injury.
Pain is subjective and difficult to quantify, as it has both affective and sensory components. The neuroanatomic basis of pain reception develops before birth, and individual pain responses are learned in early childhood. These responses are affected by social, cultural, psychological, cognitive, and genetic factors (Meldrum, 2021).
What Is Pain?
In 1979 the International Association for the Study of Pain defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” The newer 2020 definition (below) replaces terminology that relied upon a person’s ability to describe the experience to qualify as pain. Unlike the older definition, the newer definition no longer excludes infants, elderly people, and others—even animals—who cannot verbally articulate their pain.
- Pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors.
- Pain and the detection of painful stimuli are different phenomena. Pain cannot be inferred solely from activity in sensory neurons.
- Through their life experiences, individuals learn the concept of pain.
- A person’s report of an experience as pain should be respected.
- Although pain usually serves an adaptive role, it may have adverse effects on function and social and psychological well-being.
- Verbal description is only one of several behaviors to express pain; inability to communicate does not negate the possibility that a human or a nonhuman animal experiences pain.
(IASP, 2021a)
Pain alters the quality of life more than any other health-related problem. It interferes with sleep, mobility, nutrition, thought, sexual activity, emotional well-being, creativity, and self-actualization. Surprisingly, even though pain is such an important obstacle to comfort, it is one of the least understood, most undertreated, and oft-discounted problems of healthcare providers and their patients.
CONSEQUENCES OF UNTREATED/UNDERTREATED PAIN
Chronic, persistent untreated or undertreated pain prolongs systemic and chemical brain changes leading to psychological changes. Over time, these can impact brain function, resulting in changes in behavior. Chronic pain and the resulting prolonged stress response can raise blood pressure, increase respiratory and heart rates, and cause muscle tension, all of which can lead to fatigue, sleeping problems, and changes in appetite.
Activation of complex brain systems as a result of chronic pain may increase the person’s awareness of pain and decrease pain tolerance. Evidence also supports the idea that persistent pain can result in changes in the brain that control cognitive function.
Pain, and the fear of pain, can cause avoidance of both social and physical activities. Chronic pain can limit everyday activities and can affect involvement with friends and family, resulting in feelings of isolation.
Pain can cause depression, very common among those with chronic pain, or make existing depression worse; vice versa, depression can also make existing pain worse.
Research shows that uncontrolled pain has an adverse effect on the immune system and lowers the body’s ability to respond to stressful situations.
Consequences of untreated or undertreated pain can also result from pain due to damage to a nerve. This type of pain causes changes in the nervous system that contribute to the development of chronic pain long after the damage to the nerve has healed (NLM, 2021; Padgett, 2019).
| Term | Definition |
|---|---|
| (IASP, 2021a) | |
| Allodynia | Pain due to a stimulus that does not normally provoke pain |
| Analgesia | Absence of pain in response to stimulation that would normally be painful |
| Causalgia | A syndrome of sustained burning pain, allodynia, and hyperpathia after a traumatic nerve lesion |
| Dysesthesia | An unpleasant abnormal sensation, whether spontaneous or evoked |
| Hyperalgesia | Increased pain from a stimulus that normally provokes pain |
| Hyperpathia | A painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold |
| Hypoalgesia | Diminished pain in response to a normally painful stimulus |
| Neuralgia | Pain in the distribution of a nerve or nerves |
| Neuropathic pain | Pain caused by a lesion or disease of the somatosensory nervous system |
| Nociception pain | Pain arising from actual or threatened damage to non-neural tissue due to the activation of nociceptors (high-threshold sensory receptors of the peripheral somatosensory nervous system) |
| Pain threshold | Amount of pain required before individuals feel the pain; the lower the threshold, the less pain can be endured; the higher the threshold, the more pain can be endured |
| Pain tolerance level | Maximum intensity of a pain-producing stimulus that a subject is willing to accept in a given situation; the subjective experience of the individual |
| Paresthesia | An abnormal sensation whether spontaneous or evoked |
Classification of Pain
The classification of pain is complicated, and there are several different classification systems, many of which overlap. Among other characteristics, pain can be classified by duration and source.
BY DURATION
Pain is classified by duration as acute or chronic.
Acute Pain
Acute pain is protective in that it motivates a person to take action immediately. Acute pain is caused by noxious stimulation due to injury, a disease process, or the abnormal function of muscle or viscera. Acute pain begins suddenly, is usually sharp in quality, and correlates with the amount of damage. It is temporary and subsides as healing takes place. In acute pain, the central nervous system is intact, and acute pain is a symptom. Examples of causes of acute pain include:
- Surgery
- Broken bones
- Dental work
- Burns or cuts
- Labor and childbirth
There are two types of acute pain:
- Somatic pain results from superficial injury to skin and subcutaneous tissue (e.g., burns, cut, abrasions) or deep injury to muscle, bone, joint, and connective tissues (e.g., fractures, arthritis, fibrositis, rupture of muscle belly).
- Visceral pain results from injury to the internal organs (e.g., peptic ulcer, angina pectoris, renal colic).
In most instances, acute pain does not last longer than six months and disappears when the underlying cause of pain has been treated or has healed. Severe acute pain activates the sympathetic nervous system, causing diaphoresis, increased respiratory and pulse rates, and elevated blood pressure. Psychological effects of unrelieved pain can lead to anxiety and depression, and unrelieved acute pain may lead to chronic pain (Cleveland Clinic, 2022).
Chronic Pain
Chronic pain is ongoing and usually lasts longer than six months. This type of pain continues even after the injury or illness that caused it has healed. Chronic pain persists, recurs, or progresses over a long period of time and is often resistant to medical treatments. Pain signals remain active in the nervous system for weeks, months, or years. Some people suffer chronic pain even when there is no past injury or apparent body damage. Chronic pain is linked to such conditions as:
- Headache
- Arthritis and other musculoskeletal conditions
- Cancer
- Chemotherapy/radiation
- Nerve pain
- Back pain
- Fibromyalgia
- Surgical complications
Because the central nervous system may be dysfunctional, chronic pain may be considered a disease state. Chronic pain serves no biologic purpose and has no obvious end-point. If pain is associated with a disease or injury, it outlasts the normal period of healing, and the severity does not correlate with damage.
With chronic pain, stress affects the body, producing physical conditions such as tense muscles, limited ability to move about, lack of energy, and changes in appetite. Chronic pain also causes emotional effects that may include depression, anger, anxiety, or fear of reinjury (Cleveland Clinic, 2022).
BY SOURCE
The sources (causes) of pain are divided into the categories of nociceptor, neuropathic, psychogenic, and idiopathic.
Nociceptor Pain
Nociceptor pain is acute pain that results when tissue damage produces a stimulus that sends an electrical impulse across a receptor (nociceptor) by way of a nerve fiber to the central nervous system. Receptors for this type of pain are located all around the body, particularly under the skin and the internal organs. Some body tissues, such as the brain and lung, have no nociceptors, and some tissues have many.
Nociceptive pain can be divided into those that are sustained by injury to somatic tissues (bone, joints, or muscles) and those that are sustained by injury to visceral tissues. Somatic pain is often described as aching, stabbing, throbbing, or pressure-like. Visceral pain is usually described as gnawing or crampy when arising from obstruction of a hollow organ such as the bowel, and as aching or stabbing when arising from other internal organs.
Nociceptor pain is:
- Well-localized
- Worse with movement
- The result of obvious tissue injury or illness
- Caused by inflammation
- Physiologic
(Portenoy &Dhingra, 2020)
Nociplastic Pain
Nociplastic pain arises from altered nociception despite the absence of clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system. This type of pain may reflect changes in the way the nervous and immune systems function (Slater & Davies, 2021).
Neuropathic Pain
Neuropathic pain results from damage to or dysfunction of the peripheral or central nervous system rather than from stimulation of pain receptors. Mechanisms of neuropathic pain are complex and involve changes:
- At the peripheral nociceptor and nerve level
- At the dorsal root ganglion (DRG)
- In the central nervous system, nociceptive pathways, and terminal structures
Changes at the peripheral nociceptor and nerve level reduce the threshold for activation and increase the response to noxious stimuli. In chronic states, the peripheral nerve continuously triggers signals to the central nervous system.
Neurons located within the dorsal root ganglion are responsible for sensory transduction and modulation from the periphery, including pain perception. DRGs are involved in the process of chronicity, from acute to chronic pain, even after resolution of the original insult.
In the central nervous system (CNS), pain is caused by dysfunction of somatosensory pathways in the CNS. Central neuropathic pain develops only due to malfunction of the spinothalamic tract within the spinal cord, which is responsible for crude touch, pressure, and temperature.
One example of neuropathic pain is phantom limb syndrome, which can occur following an amputation and when the brain continues to receive pain messages that originally carried impulses from the missing limb (Watson, 2022).
Neuropathic pain caused by lesion or disease in the peripheral nerves may be due to:
- Traumatic brachial plexus injury
- Diabetes mellitus
- Carpal tunnel syndrome
- Postherpetic neuralgia
Neuropathic pain caused by a lesion or disease of the central nervous system may be due to:
- Central poststroke pain
- Spinal cord injury
Neuropathic pain:
- Is not well-localized
- May be burning or shooting
- May be a feeling of numbness
- Can be experienced as “pins and needles”
- May be due to tissue injury that is not evident
(IASP, 2021b)
Radicular Pain
Radicular pain is a very specific type of pain that can occur when the spinal nerve becomes compressed or inflamed. It radiates from the back and hip to the leg(s) by way of the spine and spinal nerve root. People with radicular pain may experience tingling, numbness, and muscle weakness (Beaumont Health, 2022).
Psychogenic Pain
Psychogenic pain is believed to be sustained mainly by psychological factors. It does not refer to the common idea that pain experienced by some patients is exacerbated by psychological factors, or the finding of high pain-related distress or comorbid psychiatric disease. Instead, it implies that the pain is best understood as a result of psychological processes. It is classified as a somatic symptom disorder with prominent pain, which is diagnosed on the basis of excessive thoughts, feelings, or behaviors related to pain that are distressing, impair function, and appear out of proportion to physical findings.
It must be remembered that psychogenic pain is truly experienced and is not a deception. This distinguishes it from disorders that reflect a serious mental disorder in which reports of pain may not indicate a true experience of pain, and from malingering (Portenoy & Dhingra, 2020).
Characteristics of psychogenic pain include:
- Nonlocalized pains that encompass large parts of the body
- Constant discomfort despite treatment
- Difficulty describing location, quality, and depth of pain
- Worsening pain independent of any underlying medical condition
Idiopathic Pain
Idiopathic pain, also called pain of unknown origin, is chronic pain lasting six months or longer that has no identifiable cause. Although its origin is often unknown, idiopathic pain is very real. It is also possible for this type of pain to remain long after a medical condition has healed when pain normally should have ended. Conditions in which the origin of pain may either be known or be idiopathic include:
- Fibromyalgia syndrome
- Multiple sclerosis
- “Ice-pick” headaches (pain in optic nerves)
- Irritable bowel syndrome (IBS)
- Temporomandibular joint disorder (TMJD)
(Jacques, 2021)
Physiology of Pain
Pain occurs when a noxious signal sends impulses to the spinal cord, which relays it to the brain, where it is interpreted as pain and localized. The brain determines the meaning of the signal and what should be done about it and then sends back instructions to the body about how to respond. This system is the same for everyone, but the sensitivity and efficacy of these brain circuits determines how much the person feels and how the person copes with pain.
In response to a noxious stimulus, an involuntary and nearly instantaneous movement (reflex) occurs. The path taken by the nerve impulses in a reflex is called a reflex arc. Most sensory neurons do not pass directly to the brain but synapse in the spinal cord. This allows physical actions to occur relatively quickly by activating spinal motor neurons without the delay of routing signals through the brain. The brain, however, will receive sensory input while the reflex action occurs (Medicine LibreTexts, 2020).
TISSUE DAMAGE
Receptors (nociceptors) located in the skin and other tissues are nerve fibers with endings that can be excited by three types of stimuli—mechanical, thermal, and chemical. When tissue is damaged, there is an immediate release of inflammatory chemicals (called excitatory neurotransmitters) such as serotonin, histamine, and bradykinin (a powerful vasodilator). Increased blood in the area causes the injured area to swell, redden, and become tender. The bradykinin stimulates the release of prostaglandins and substance P, a potent neurotransmitter that enhances the movement of impulses across nerve synapses (Meldrum, 2021).
MEDIATION
Pain is mediated (caused) by two major types of nociceptor nerve fibers (A-delta fibers and C fibers), which are the nerve endings of the first-order neurons in the pain pathway.
The A-delta fibers are the larger of the two and the most rapidly conducting (12–30 m/sec) because of their thin myelin covering. They respond to thermal, mechanical, and chemical stimuli, and are responsible for the sharp, well-localized pain that is first perceived.
C fibers are smaller, and because they are unmyelinated, impulse signals are slower (0.5 m/sec). C fibers respond to chemical, mechanical, and thermal stimuli, and are associated with the longer-lasting, burning, dull, and poorly localized sensations that follow the first sensation of pain.
Impulse signals travel via the spinal nerves to the spinal cord, where they synapse with second-order neurons in the dorsal horn of the spinal cord. The second-order neurons then cross over to the other side of the spinal cord before ascending to the opposite side of the brain from that part of the body sending the impulse.
Two different pathways—the spinothalamic and spinoreticular tracts—transmit impulses to the brainstem and thalamus. Spinothalamic input is thought to affect the conscious sensation of pain, and the spinoreticular tract is thought to affect the arousal and emotional aspects of pain (Meldrum, 2021).
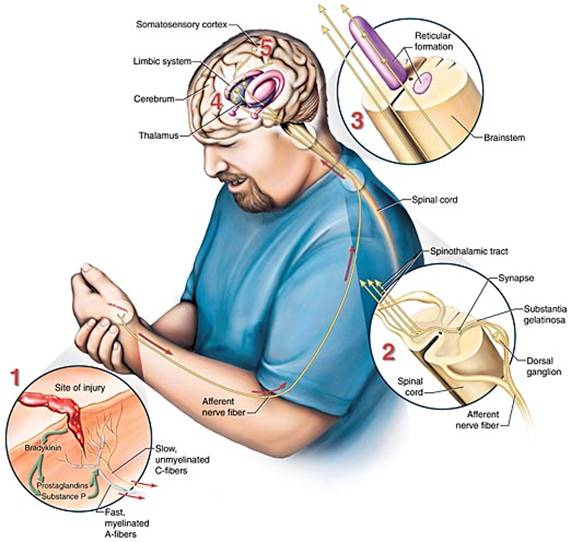
Neurologic transmission of pain stimuli. (Source: Jason M. Alexander. © 2005, Wild Iris Medical Education.)
PERCEPTION
In the cortex of the brain, a sensory strip receives the nerve impulse. This sensory strip, the somatosensory homunculus (Latin for “little man”), is basically a brain map of the body. Nerve impulses travel to the area in the homunculus that corresponds to the part of the body the signal is coming from. All information from each body part (e.g., the finger) end up in one specific area of the homunculus after entering the sensory strip in the cortex of the brain (Kahn Academy, 2022).
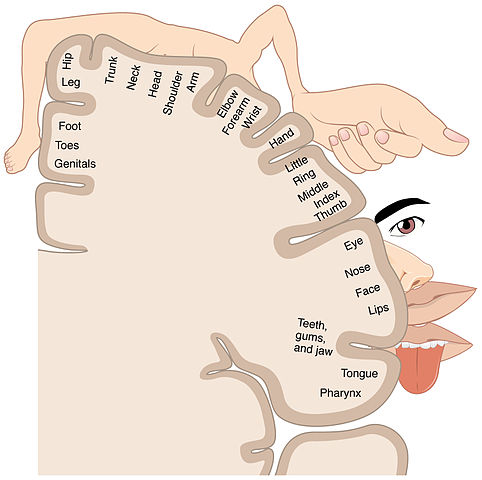
Somatosensory homunculus, or sensory strip. (Source: BodyParts3D, © The Database Center for Life Science.)

The somatosensory homunculus (“little man”) shows a distorted image of the human body because the size of each region of the map is related to the density of sensory receptors in the body part. (Source: OpenStax College, Anatomy & Physiology.)
Perception of pain results from the brain’s processing of new sensory input with existing memories and emotions. When the message is received in the homunculus, the brain recognizes the pain stimulus and interprets its significance. Several factors can affect how the brain interprets the pain, including:
- Emotional and psychological states
- Memories of previous pain
- Upbringing
- Expectations of and attitudes toward pain
- Beliefs and values
- Age
- Gender
- Social and cultural influences
(Meldrum, 2021)
MODULATION
Pain modulation, the process of alterations in pain signals along the transmission pathway of pain, explains why individuals respond to the same stimulus differently. Once the brain perceives the pain and determines its meaning, it sends messages downward to affect the sensitivity and behavior of nerves. The body releases neuromodulators, including endogenous opioids (endorphins and enkephalins), serotonin, norepinephrine, and gamma aminobutyric acid. These chemicals hinder the transmission of pain and help produce an analgesic, pain-relieving effect.
The descending paths of the efferent fibers extend from the cortex down to the spinal cord and may influence pain impulses at the level of the spinal cord. This system provides a necessary survival function, as it regulates fear and anxiety, allowing the pain experience to be altered according to the situation rather than having pain dominate (Dafny, 2020).
GATE-CONTROL THEORY
In 1965 Melzack & Wall suggested that there is a gate or control system in the dorsal horn of the spinal cord through which all information regarding pain must pass before reaching the brain. An open gate means transmission cells (t-cells) can carry signals to the brain, where pain is perceived. A closed gate stops the transmission, and no pain signal is sent to the brain.
The substantia gelatinosa (SG) in the dorsal horn of the spinal cord controls whether the gate is open or closed. The SG has both excitatory (SG+) and inhibitory (SG-) synapses with the t-cells. There are three kinds of neurons that send signals to the SG. Two of them, A-delta fibers and C fibers, transmit pain signals and open the gate. The third type of neurons, A-beta fibers, responds to nonpainful stimuli such as touch, inhibits the transmission of pain signals, and closes the gate.
An understanding of how this gate-control theory works can be realized by considering a bump to the elbow. When the injury to the elbow occurs, the A-delta fibers are activated, followed by activation of the C fibers, and the pain signal is transmitted. By rubbing the bump, the large, fast- conducting A-beta fibers are stimulated. This stimulation is nonpainful, and the signal is transmitted faster than the A-delta and C fiber signals. The A-beta transmission reaches the SG-, closes the gate, and inhibits the further transmission of pain.
Because the perception of pain has a large cognitive component (e.g., distraction, thoughts, emotions), fast-conducting fibers from the thalamus and cerebral cortex areas of the brain can diminish pain by sending an inhibitory signal through the SG and thus close the gate (Weber, 2022).
Factors That Influence the Experience of Pain
The experience of pain is influenced by both physiologic and psychosocial factors, all of which clinicians must consider in pain management.
PHYSIOLOGIC FACTORS
Physiological factors that influence pain include age, gender, genetic makeup, and stress response.
Age
Multiple studies have demonstrated that age-related changes in peripheral and central nervous systems affect all levels of pain processing. The main findings from studies on pain sensitivity include an increased threshold and decreased tolerance. These changes begin in middle-age, when the prevalence of chronic pain is starting to peak.
As age increases, there is a decrease in somatosensory perception related to the loss of nociceptors and mechanoreceptors and to reduced blood flow to the skin. Neuronal fiber loss and reduced conduction velocity are also associated with reduced sensitivity. Peripheral nociceptors contribute little to the development of chronic pain in the older adult, and this explains why peripherally acting analgesics (such as NSAIDs) can have little effect in an older population. As the individual ages, the processes by which the body alters a pain signal (modulation) as it is transmitted along the pain pathways appears to become less efficient.
There is a lack of evidence that examines chronic pain in children and adolescents, however, available literature suggests that older patients have a higher prevalence of chronic pain than young patients.
At the brain level, despite a significant reduction in grey and white matter typical of the aging process, functional MRI studies reveal that the brain activation in response to painful stimuli and central processing pathways remain unchanged even in extreme age and in moderate cognitive impairment. Significant changes in brain structure have been found in older people with chronic pain, but it is unclear whether these changes are caused by chronic pain or are a predisposing factor to the development of more severe pain perception (Tinnirello et al., 2021; Mills et al., 2019).
Gender
Women appear to suffer pain more often and with greater emotional stress than do men, but some evidence shows that women may cope with severe pain more effectively than men. Men are less likely to report or experience chronic pain than women, and girls are more likely to report having pain in multiple sites than boys.
Studies about how gender (role) and sex (biological) differences are related to the way men and women experience pain have been carried out, which find that women have lower pain thresholds and tolerance and are more likely to experience greater intensity and unpleasantness with pain.
Currently, there is insufficient information about the mechanisms behind these sex-specific differences in pain perception and pain prevalence, but there is some evidence for the role of estrogen and genetics. The fluctuating nature of female hormones may amplify the body’s perception of pain. When estrogen levels are low during the menstrual cycle or after menopause, pain receptor activity is elevated, causing the body to experience more pain.
Studies looking at biological sex have found that at puberty, the rate of pain rises more in girls than boys. And as women age and enter menopause, hormonal levels change and sex differences in chronic pain rates begin to disappear (Dance, 2019).
Genetic Makeup
Studies to date find that there are over 200 genes involved in pain processing and perception. These genes:
- Affect pain independently or jointly, by interaction with environmental factors
- Affect susceptibility to diseases that may cause pain
- Affect susceptibility to more severe and more chronic pain
- Reduce or protect from pain
Genetics can explain heritability in:
- Low back pain (68%)
- Neck pain (58%)
- Migraines (39%–58%)
- Menstrual pain (55%)
- Chronic postsurgical pain (50%)
(Ratka, 2020)
Stress Response
Pain acts as a survival signal for the brain, telling the brain to prepare for “fight or flight.” In response, the brain changes both physically and chemically. This is coupled with changes in the body, such as increased heart rate, prioritization of blood flow to the muscles, and other stress responses, including neural, endocrine, and behavioral changes. Some people are more sensitive and react to this stress, while others are more resilient. Normally, the body resolves these changes and returns to normal following temporary pain. Chronic pain, however, presents different problems.
Chronic persistent pain prolongs the systemic and chemical brain changes, which in turn leads to psychological changes. Over time, these changes can impact brain function, resulting in changes in behavior. This chronic stress, however, is not limited to psychological effects alone. Chronic pain and the resulting prolonged stress response can lead to cardiac issues and gastrointestinal changes, among other things (Padgett, 2019).
PSYCHOSOCIAL FACTORS
Pain perception is the result of the brain’s processing of new sensory input with existing memories and emotions. Childhood experiences, cultural attitudes, heredity, and gender contribute to the development of pain perception and response to pain. Some people may be physiologically able to withstand pain better than others, and cultural factors rather than heredity usually account for that ability.
Depression and anxiety can lower pain thresholds. Anger and excitement can obscure or lessen pain temporarily. Feelings of emotional relief can also lessen a painful sensation (Meldrum, 2021).
Personality
Conflicting evidence exists regarding the role of personality on the variability of pain perception. There are numerous studies, however, that show relationships between sensitivity to pain and the personality traits of neuroticism, extraversion, and openness to experience (see below). Individuals who are more sensitive to pain tend to be high in neuroticism while low in openness to experience and extraversion The personality trait of neuroticism is considered to be among the most significant moderators of pain.
- Neuroticism, a negative personality trait, is characterized by sadness, moodiness, and emotional instability. Individuals with neuroticism exhibit a short pain tolerance and a high pain intensity.
- Openness to experience, a positive personality trait, is characterized by being more willing to embrace new things, fresh ideas, and novel experiences. Persons with this personality trait engage in self-examination and have lower sensitivity to pain.
- Extraversion, a positive personality trait, is characterized by being a “people person,” directing energies toward other people and the outside world. These individuals exhibit lower pain sensitivity.
(Eisenberg, 2021; Bar-Shalita & Cermak, 2020)
Pain Appraisal and Beliefs
Pain appraisal refers to the meaning ascribed to pain by an individual. Primary appraisal involves evaluation of the significance of the pain as either a threat or irrelevant, and secondary appraisal involves evaluation of the controllability of pain and one’s coping resources. Beliefs refer to assumptions about reality that shape how the person interprets events.
Appraisal and beliefs about the meaning of pain can have a strong impact on an individual’s response to pain. If a pain signal is interpreted as harmful, it may be perceived as more intense or more unpleasant and evoke more escape or avoidance behaviors.
Pain appraisal and pain beliefs are also determinants of adjustment to chronic pain. Pain that is viewed as a signal of damage, leading to disability, uncontrollable, and a permanent condition has been shown to affect an individual’s responses (Ballantyne et al., 2019).
Fear and Catastrophizing
Pain catastrophizing is an exaggerated, negative cognitive and emotional orientation toward actual or anticipated pain experiences. Catastrophizing has been associated with an increased perception of severity and disability in both acute and chronic pain among persons with many different pain diagnoses. Catastrophizing also alters perception of noxious stimulation.
People who experience chronic pain often anticipate that specific activities will increase pain or induce further injury, and these fears may contribute to avoidance of activity and subsequently greater physical deconditioning, emotional distress, and ultimately, greater disability (Ballantyne et al., 2019).
Emotions
Emotion and pain interact in several ways. Emotional distress may predispose a person to experience pain, be a cause of symptoms, be a modulating factor amplifying or inhibiting the severity of pain, be a consequence of persistent pain, or be a perpetuating factor. Emotional distress is commonly observed in people with chronic pain.
Anxiety is common for patients with pain. Up to 45% of patients with chronic pain screen positive for an anxiety disorder. Those with chronic pain who have comorbid anxiety may have a lower pain tolerance, be more prone to medication side effects or fearful of having side effects, and be more fearful of pain itself.
Up to 50% of patients with chronic pain experience depression, and on average, 65% of depressed individuals also report pain symptoms. There is evidence of a strong association between chronic pain and depression, but evidence is lacking as to whether chronic pain causes depression or depression causes chronic pain (Ballantyne et al., 2019).