EMERGENCY DEPARTMENT STROKE EVALUATION AND MANAGEMENT
EMS delivery ideally involves transporting the patient to the nearest facility with appropriate stroke resources. Acute stroke treatment protocols involve specialized knowledge and practical experience. However, the facilities, equipment, and personnel for acute stroke management are expensive and are not available at most hospitals.
Emergency department care addresses these links in the stroke chain of survival:
- 5. Data: Obtaining laboratory results; performing physical and neurologic exams, and brain imaging
- 6. Decision: Determining appropriate treatment
- 7. Drug: Administering drug therapy if appropriate
Types of Stroke Care Facilities
The objective of stroke centers is to improve the quality and organization of acute stroke care. Certifying organizations reward designated facilities for following evidence-based guidelines and having the ability to provide basic and advanced levels of care. Some of these organizations include The Joint Commission (TJC) and the Healthcare Facilities Accreditation Program (HFAP), with TJC being the oldest of the two. Each agency has different outcome measures including healthcare team experience, patient volume, research, and survey frequency.
The Joint Commission (TJC) classifies hospitals into four categories based upon the level of care they are able to provide for stroke patients:
- Acute stroke-ready hospital
- Primary stroke center
- Thrombectomy-capable stroke center
- Comprehensive stroke center
ACUTE STROKE-READY HOSPITAL (ASRH)
Acute stroke-ready hospitals tend to be smaller hospitals located in rural and suburban areas. An acute stroke-ready hospital differs from a non–stroke center in that they have around-the-clock access to stroke expertise (either by telephone or in person) and the ability to administer IV thrombolytics prior to transferring a patient for more advanced care.
TJC certification for these facilities requires that they provide the following:
- A medical program director with sufficient knowledge of cerebrovascular disease
- Stroke protocols and an acute stroke team available 24 hours/7 days per week and at bedside within 15 minutes
- Initial assessment performed by an emergency department physician, nurse practitioner, or physician assistant
- CT, MRI (if used), and laboratory capability 24/7
- Access to a neurologist 24/7 in person or by telemedicine
- Neurosurgical services available within three hours (through transfer)
- IV thrombolytic administration capability
- Transfer protocols with one primary stroke center (PSC) or comprehensive stroke center (CSC) (see below)
- Telemedicine available within 20 minutes
- Required ED staff education a minimum of twice a year, core team at least 4 hours annually
- Collaborative relationship with local EMS, providing educational opportunities to prehospital personnel
- Use of three inpatient and two outpatient standardized measures to evaluate clinical performance
Following initial certification, recertification is required every two years following an onsite review (TJC, 2021).
PRIMARY STROKE CENTER (PSC)
To be certified as a primary stroke center, an emergency department (or its hospital) must meet the following criteria:
- A stroke team available 24/7, at bedside within 15 minutes
- A stroke unit or designated beds for the acute care of stroke patients
- Initial assessment completed by an emergency department physician
- CT, MRI, laboratory, CT angiography, MRA (magnetic resonance angiography) availability 24/7; at least one modality for cardiac imaging when needed
- Neurologist available 24/7 in person or via telemedicine
- Access to neurosurgical services within two hours; operating room (OR) availability 24/7 in a PSC that provides neurosurgical services
- Telemedicine available
- Treatment with IV thrombolytics and medical management of stroke
- Transfer protocols for neurosurgical emergencies
- Required ED staff education a minimum of twice a year, core team at least 8 hours annually
- Provision of education to prehospital personnel and a minimum of two stroke education activities per year to the public
- Use of eight standardized measures to evaluate clinical performance
Following initial certification, recertification is required every two years following an onsite review (TJC, 2021).
TELESTROKE CONSULTATION
Ideally, the treatment of all acute strokes is provided in PSCs or higher-level facilities, but many areas of the country are far from such centers. One way to extend the range of acute stroke treatment, especially the administration of thrombolytic agents, into areas far from stroke specialists is by using video teleconsultation, or telestroke.
Telestroke (telemedicine) is a two-way videoconference between distant stroke-care specialists (neurologists) and local emergency medicine doctors to recommend diagnosis and treatment that can be given in the local community. This avoids the need for transfer to another medical center, thereby reducing the delay between recognition of stroke and appropriate treatment.
Like a direct onsite consultation, physicians and patients communicate using digital video cameras, internet telecommunications, robotic telepresence, smartphones, tablets, and other technology.
In telestroke, many people work together as a team, including a program manager, clinical coordinator, vascular neurologists, neurosurgeons, and radiologists at the distant site, and emergency medicine physicians and other staff at the originating site. Radiology technicians, informational technology staff, researchers, nurses, nurse practitioners, and other staff also are important members of the team (Mayo Clinic, 2020).
THROMBECTOMY-CAPABLE STROKE CENTER (TSC)
A thrombectomy-capable stroke center is a facility that has performed mechanical thrombectomy and post-procedure care for at least 15 patients with ischemic stroke in the past 12 months or at least 30 patients over the past 24 months. The requirements for this certification include:
- A program medical director with a neurology background and ability to provide clinical and administrative guidance to program
- A dedicated neuro-intensive care unit or designated intensive care beds for complex stroke patients available 24/7; onsite critical care coverage 24/7
- CT, MRI, labs, CTA, MRA available 24/7; other cranial and carotid duplex ultrasound, TEE (transesophageal echocardiogram) available as indicated
- A neurologist accessible 24/7 in person or via telemedicine and written call schedule for attending physicians available 24/7
- Neurosurgical services available within 2 hours; OR available 24/7
- Treatment capability that includes IV and IA thrombolytics, mechanical thrombectomy
- Education for nurses and other ED staff (2 hours annually), and for stroke nurses and core stroke teams (8 hours annually)
- Provision of educational opportunities to prehospital personnel, and at least two stroke education activities annually to the public
- Having standardized measures for clinical performance: eight inpatient (STK) measures and five ischemic comprehensive stroke (CSTK) measures, for a total of 13
(TJC, 2021)
COMPREHENSIVE STROKE CENTER (CSC)
Comprehensive stroke center certification is available only in Joint Commission–accredited acute care hospitals. Organizations seeking CSC certification must meet all of the following eligibility requirements:
- Program medical director with extensive expertise available 24/7
- Dedicated neuro-intensive care unit beds for complex stroke patients and neuro-intensivist coverage 24/7
- CT, MRI, labs, CTA, MRA, catheter angiography 24/7; other cranial and carotid duplex ultrasound; TTE (transthoracic echocardiogram) as indicated
- Neurologist who meets emergent needs of multiple complex stroke patients; written call schedule for attending physicians providing availability 24/7
- Neurosurgic service available 24/7 (neurointerventionist, neuroradiologist, neurologist, neurosurgeon)
- Treatment: IV thrombolytics; endovascular therapy; microsurgical neurovascular clipping of aneurysm; neuroendovascular coiling of aneurysms; stenting of extracranial carotid arteries; carotid endarterectomy
- Protocols for receiving transfers and circumstances for not accepting transfers
- Staff education for nurses and other ED staff (2 hours annually) and stroke nurses and core stroke team (8 hours annually)
- Sponsor a minimum of two public education opportunities annually; present a minimum of two educational courses annually for internal staff or individuals external to the CSC (e.g., hospitals and licensed independent practitioners)
- Participate in stroke research approved by the institutional review board
- Use of eight core inpatient stroke measures and 10 comprehensive stroke measures, for a total of 18, to evaluate clinical performance
(TJC, 2021)
MOBILE STROKE UNIT (MSU)
Mobile stroke units are based on the theory that bringing stroke treatment to patients could reduce prehospital delays, improve thrombolysis times, and subsequently improve patient accounts. As of 2021, there were 20 MSUs in the United States, and it was found that patients treated by MSU are 2.5 times more likely to have a good outcome. Mobile stroke units bring hospital-grade stroke diagnosis and treatment straight to the patient, saving on average 36 minutes.
An MSU is an ambulance equipped with all the traditional medications, tools, and resources used to treat stroke patients in a hospital setting. Most importantly, MSUs house portable CT scanners capable of 8-slice image acquisition that is comparable to in-hospital CT image quality. MSUs are equipped with lead-reinforced walls and floors, lead vests and personal radiation detectors are worn by staff, and protocols are in place to protect staff members and the public from radiation.
The team can vary per MSU program but always includes EMS personnel, an appropriately credentialed clinician responsible for tissue plasminogen activator (tPA) treatment decisions, and a technologist to operate the CT scanner. Others may include a vascular neurologist either on-board or via telemedicine and a registered nurse with specialized stroke training (Ehntholt et al., 2020; Neff, 2021).
STROKE PROTOCOLS
When a potential stroke patient enters any ED, staff must begin a protocol that can lead directly to the administration of a thrombolytic drug at the present hospital or at a stroke center. The main goals are rapid access to thrombolysis for ischemic stroke patients and stabilization and rapid admission to a stroke unit for all stroke patients.
Upon arrival, a typical protocol to accomplish the goal of rapid treatment includes:
- Immediate emergency department triage to high-acuity area
- Prompt emergency department evaluation
- Stroke team mobilization
- Lab studies and brain imaging
- Diagnosis and determination of most appropriate therapy
- Administration of drugs/treatment/interventions
- Timely admission to stroke unit, ICU, or transfer
(Physiopedia, 2022a)
Triage
Time-to-treatment is critical. Therefore, patients with suspected acute stroke are assigned the same high priority as patients with acute myocardial infarction or serious trauma, regardless of the severity of their neurologic deficits.
About one half of all stroke patients will not enter the emergency department by ambulance. The ED registration staff must be trained to recognize signs of possible stroke. The front desk nurse should have a written stroke-recognition checklist. This will ensure that any triage nurse can quickly channel potential stroke victims into the ED’s stroke protocol.
IDENTIFICATION OF A POTENTIAL STROKE VICTIM
The Emergency Severity Index (ESI) is a five-level emergency department triage algorithm that provides clinically relevant stratification of patients into five groups, from 1 (most urgent) to 5 (least urgent), on the basis of acuity and resource needs, including labs, ECG, X-rays, CT, MRI, ultrasound-angiography, IV hydration, IV or IM medications, specialty consultation, and simple and complex procedures (AHRQ, 2020).
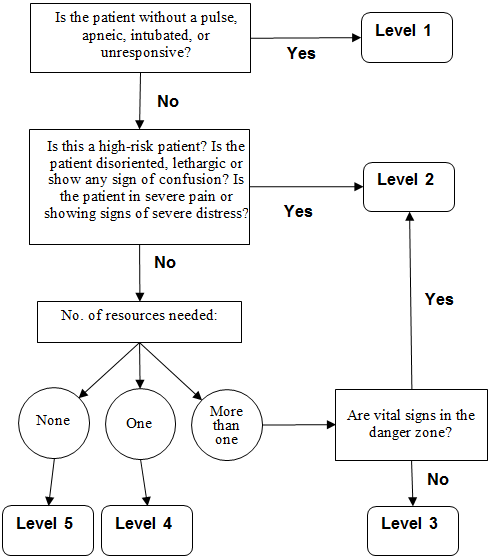
A 5-level ESI triage algorithm. (Source: Adapted from Gilboy et al., 2011.)
This algorithm triages patients based on the severity of symptoms.
- Level 1 represents a patient who has no pulse, may be intubated or unable to breathe on their own, and may be unresponsive to noxious stimuli (P/U on APVU scale) or to verbal commands. Immediate life-saving intervention such as resuscitation is required.
- Level 2 (where stroke patients should be placed) is a high-risk, emergency situation. The recommendation here is to imagine the hospital had “one last bed.” The stroke patient should get priority. This is true whether or not the patient is confused, lethargic, or disoriented and whether or not the patient is in pain.
- Levels 3, 4, and 5 are least urgent and would not apply to a stroke patient. They are dependent upon the number of resources required to provide appropriate care.
(AHRQ, 2020)
STROKE CENTER TIME TARGETS
Stroke centers are dedicated to quick, efficient care. The recommended time targets for key steps in the management of acute stroke are as follows:
- From the door to a physician: 10 minutes, which includes:
- Medical stabilization
- History, time last known well (LKW)
- Eligibility for tPA, etc.
- Focused exams
- Examination using appropriate stroke scale
- Initiation of lab work
- From physician to neurologic expertise: 15 minutes or less
- From the door to a completed CT or MRI: 25 minutes or less
- From the door to the reading of the CT or MRI scan by a specialist: 45 minutes or less
- From the door to treatment: 60 minutes or less
- From the door to admission to a monitored bed: 3 hours
(Lahouti, 2021)
TIME SHEET DOCUMENTATION DURING TRIAGE
For patients with an acute onset of neurologic signs, triage nurses complete the following:
- A stroke recognition checklist
- A time sheet documenting:
- Time of onset of symptoms, or time when the patient was LKW
- Time of the patient’s arrival in the ED
- Time goal for the initial provider’s assessment (i.e., 10 minutes after the patient’s arrival at the ED)
- Time goal for a completed CT/MRI scan (i.e., 25 minutes after the patient’s arrival)
- Time windows for the recombinant tissue plasminogen activator (rtPA) treatment of eligible patients
- ED goal (e.g., within 60 minutes of the patient’s arrival)
- 3-hour time window after onset of symptoms
- 4.5-hour time window in well-screened patients who are at low risk for bleeding
- 4.5- to 6-hour time window, evaluate candidate for mechanical thrombectomy
- Door to admission time of 3 hours after arrival for all patients
(ACLS, 2020; Filho & Samuels, 2022)
The time sheet then follows the patient to keep providers, nurses, and technicians on schedule.
ROLE OF THE EMERGENCY DEPARTMENT NURSE
The emergency department nurse who initially evaluates the patient:
- Notifies CT to anticipate an emergent CT scan
- Within 10 minutes of arrival, assesses vitals and provides oxygen if patient is hypoxemic
- Obtains IV access (if not done by EMS)
- Checks glucose (unless EMS glucose value is already known)
- Activates the stroke team
- Anticipates orders for:
- CT without contrast or MRI to rule out ICH or nonstroke lesions
- CT with contrast head/neck
- Laboratory tests for cardiac-specific troponin and chemistry panel
- Coagulation studies
- Arterial blood gas analysis to assess for hypoxemia
- 12-lead EKG to rule out myocardial infarction (MI) and atrial fibrillation
- Electroencephalogram (EEG) to rule out ongoing seizures
- Chest X-ray if clinically indicated
(ACLS, 2020; OHSU Healthcare, 2022)
MOBILIZATION OF THE HOSPITAL’S STROKE TEAM
When a potential stroke patient has been identified, a stroke page is initiated from the incoming EMS vehicle or from the ED triage nurse. The stroke team responds to the page within 5 minutes and reviews the patient’s history, establishes a timeline of symptom onset, and performs a neurologic examination using either the NIH Stroke Scale or the Canadian Neurological Scale.
If the patient is determined to have an ischemic stroke, thrombolytics and/or thrombectomy will be considered. If CT or MRI subsequently shows intracranial hemorrhage (subarachnoid or intracerebral), immediate neurology and neurosurgery consults will be obtained (ACLS, 2020).
CASE
Eleanor, a 62-year-old African American female patient, arrives to the emergency department accompanied by her daughter. Eleanor presents with sudden onset of left-eye blindness beginning 30 to 45 minutes ago while she was at home reading a magazine. Her daughter called 911 for immediate transport. Eleanor says it was as if “someone had dropped a gray curtain over my left eye” but that her vision is improving.
The nurse in the ED, Joan, asks the patient if she has had a headache, weakness, dizziness, tingling, fatigue, or slurred speech in the past. Beyond occasional headaches, Eleanor denies any of these symptoms and adds that this blindness has never happened to her before. Eleanor’s health history reveals that she has well-controlled type 2 diabetes and hypertension, with untreated hyperlipidemia that was recently diagnosed.
Eleanor’s medications include metformin (Glucophage), 1,000 mg, by mouth twice daily; lisinopril (Zestril), 5 mg, by mouth daily; and hydrochlorothiazide (Esidrix), 25 mg, by mouth daily. Eleanor was previously on estrogen replacement therapy for eight years post hysterectomy. Her pertinent family history includes a mother who had a cerebrovascular event at age 82 years.
Based on Eleanor’s symptoms, medical history, and family history, the nurse immediately consults with the ED physician and alerts the stroke team. The nurse also reassures Eleanor and her daughter that they were right to call 911.
Stabilization of Comorbid Medical Problems
Within 10 minutes of arrival, a general examination is done to identify other potential causes of the patient’s symptoms and coexisting comorbidities or issues that may impact the management of a stroke.
IDENTIFY AND TREAT MEDICAL PROBLEMS
A quick but thorough examination is done to assess for circulation, airway, breathing, and vital signs and to medically stabilize any problems the patient may have in addition to the stroke.
- For oxygen saturation <94%, give O2 via nasal cannula at 2–3 L/min. Supplemental oxygen is not recommended in nonhypoxic patients with AIS.
- Hypoperfusion and hypovolemia should be corrected.
- Patients with elevated blood pressure (BP) who are eligible for fibrinolytic therapy should have BP carefully lowered to <185 mmHg systolic and <110 mmHg diastolic before IV fibrinolytic therapy is begun.
- Sources of elevated temperature >100.4 °F should be identified and treated. Antipyretic medications should be administered if indicated.
- Treat hyperglycemia to achieve blood glucose levels in the range of 140–180 mg/dL.
- Treat hypoglycemia (<60 mg/dL) in all patients with AIS.
- Establish IV access if not yet done. Patients eligible for rtPA therapy will need a minimum of two IV sites, one for IV fluids and/or IV medications and one dedicated to rtPA administration.
- Establish continuing cardiac monitoring.
- If the patient is alcoholic or malnourished, thiamine should be given.
(ACLS, 2020; Powers et al., 2019)
Stroke Diagnostic Studies
A blood glucose laboratory test must be measured in all patients. Coagulation studies (international normalized ratio [INR], activated partial thromboplastin time [aPTT], and platelet count) may also be required if there is suspicion of coagulopathy. Because of the very low risk of unsuspected abnormal findings, fibrinolytic treatment should not be delayed while waiting for testing if there is no reason to suspect abnormal results (Powers et al., 2019).
Additional laboratory tests are tailored to the individual patient and may include the following:
- Cardiac biomarkers
- Toxicology screen
- Fasting lipid profile
- Erythrocyte sedimentation rate (ESR)
- Pregnancy test
- Antinuclear antibody (ANA)
- Rheumatoid factor
- Homocysteine level
- Rapid plasma reagent (RPR)
A urine pregnancy test should be obtained for all women of childbearing age with stroke symptoms. The safety of the fibrinolytic agent recombinant tissue-type plasminogen activator (rtPA) in pregnancy has not been studied in humans (Jauch, 2022).
Medical History
The medical history should include the patient’s chief complaint and the history of the present illness. The most important piece of historical data, however, is the time of symptom onset or last known well (LKW). The history should include all symptoms the patient has experienced, as well as the time and sequence of each of them. Obtaining this history may require interviewing a family member and/or a witness.
A medical history for stroke patients includes a review of systems, eliciting the following information:
- Hypertension
- Diabetes mellitus
- Tobacco use
- High cholesterol
- History of coronary artery disease, coronary artery bypass, or atrial fibrillation
In younger patients, elicit a history of:
- Recent trauma
- Coagulopathies
- Illicit drug use (especially cocaine)
- Migraines
- Oral contraceptive use
Nausea, vomiting, headache, and a sudden change in the patient’s level of consciousness helps distinguish ischemic from hemorrhagic stroke (Jauch, 2022).
Patient Examination
Patient examination includes a focused physical examination, a neurologic examination, and a formal stroke assessment.
FOCUSED PHYSICAL EXAMINATION (WITH ECG)
The purpose of the focused general physical is to:
- Detect extracranial causes of stroke symptoms
- Distinguish stroke from stroke mimics
- Determine and document the degree of neurologic deficit using the NIH Stroke Scale in order to determine severity and possible location of the stroke and for future comparison
- Localize the lesion
- Identify comorbidities
- Identify conditions that can influence treatment decisions, such as recent surgery or trauma, active infection, or active bleeding
The physical examination must include all major organ systems, including a careful head and neck exam for signs of trauma, infection, and meningeal irritation. Vital signs can point to impending clinical deterioration and may assist in narrowing the differential diagnosis.
A search for the cardiovascular causes of stroke requires examination of the following:
- Ocular fundi for retinopathy, embolic, or hemorrhage
- Heart for arrhythmias, such as atrial fibrillation, gallop, or murmur
- Peripheral vascular including palpation of carotid, radial, and femoral pulses, and auscultation for carotid bruit
- Unequal pulses or blood pressures in the extremities, which may indicate the presence of an aortic dissection
(Jauch, 2022)
NEUROLOGIC EXAMINATION
A brief but accurate neurologic exam should be done with the goals of:
- Confirming the presence of stroke syndrome
- Distinguishing stroke from stroke mimics
- Establishing a neurologic baseline to assess improvement or deterioration of condition
- Establishing stroke severity to assist in prognosis and therapeutic selection
The essential components of the neurologic examination include:
- Cranial nerves
- Motor function
- Sensory function
- Cerebellar function
- Gait
- Expressive and receptive language capabilities
- Mental status and level of consciousness
The skull and spine should be examined for signs of meningismus (neck stiffness, headache, and other symptoms suggestive of meningeal irritation) (Jauch, 2022).
FORMAL STROKE ASSESSMENT
American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend all potential stroke victims be assessed using the NIH Stroke Scale (NIHSS). This is a measure of the severity of neurologic deficits and can be used to objectively monitor the improvement or deterioration of the stroke. The NIHSS scale is designed to be simple, valid, and reliable and can be administered consistently by physicians, nurses, or therapists.
Standardized stroke assessment tools do not replace a neurologic exam. Instead, the stroke scale is an efficient way to objectively determine the severity and possible location of the stroke. NIHSS scores are helpful in identifying patients who would likely benefit from fibrinolytic therapy and those at greater risk of hemorrhagic complications of fibrinolytic use.
The NIHSS focuses on six major areas of the neurologic examination: These include:
- Level of consciousness
- Visual function
- Motor function
- Cerebellar function
- Sensation and extinction (formerly known as neglect)
- Language
The NIHSS is a 42-point scale. Patients with minor strokes usually have a score of <5. An NIHSS score >10 correlates with an 80% likelihood of proximal vessel occlusions.
| Instructions | Scale | Score |
|---|---|---|
| (Jauch, 2022) | ||
| Level of consciousness (LOC) observed | Alert | 0 |
| Drowsy | 1 | |
| Stuporous | 2 | |
| Comatose-unresponsive | 3 | |
| Orientation questions: What month is it? What is your age? | Answers both correctly | 0 |
| Answers one correctly | 1 | |
| Answers both incorrectly | 2 | |
| Response to commands: Open and close eyes. Grip and release nonparetic hand. | Performs both tasks correctly | 0 |
| Performs one task correctly | 1 | |
| Performs neither | 2 | |
| Best gaze: Follow my finger with your eyes. | Normal horizontal movements | 0 |
| Partial gaze palsy | 1 | |
| Forced deviation | 2 | |
| Visual fields | No visual field deficit | 0 |
| Partial hemianopia | 1 | |
| Complete hemianopia | 2 | |
| Bilateral hemianopia | 3 | |
| Facial palsy: Show teeth. Raise eyebrows. Squeeze eyes shut. | Normal, symmetrical | 0 |
| Minor facial weakness | 1 | |
| Partial facial weakness | 2 | |
| Complete unilateral palsy | 3 | |
| Motor arms: Extend arms and hold 10 seconds, preferably with palm facing up (each arm tested and scored separately). | No drift | 0 |
| Drift before 10 seconds | 1 | |
| Falls before 10 seconds | 2 | |
| Falls, no effort against gravity | 3 | |
| No movement | 4 | |
| Motor legs: In supine position, raise leg 30 degrees (each leg tested and scored separately). | No drift | 0 |
| Drift before 5 seconds | 1 | |
| Falls before 5 seconds | 2 | |
| Falls, no effort against gravity | 3 | |
| No movement | 4 | |
| Cerebellar testing: Limb ataxia (finger-nose-finger and heel-shin tests on both sides). | Absent | 0 |
| Ataxia in one limb | 1 | |
| Ataxia in two limbs | 2 | |
| Sensory: Pinprick to face, arm, leg. | No sensory loss | 0 |
| Mild sensory loss | 1 | |
| Severe sensory loss | 2 | |
| Sensory: Extinction. Double simultaneous test. | No neglect | 0 |
| Partial neglect (1 sensory modality lost) | 1 | |
| Complete neglect (2 modalities lost) | 2 | |
| Best language: Ask the patient to name items and describe pictures. | No aphasia | 0 |
| Mild to moderate aphasia | 1 | |
| Severe aphasia | 2 | |
| Mute, global aphasia | 3 | |
| Dysarthria: Assess speech clarity to “mama, baseball, huckleberry, tip-top, fifty-fifty.” | Normal articulation | 0 |
| Mild to moderate dysarthria | 1 | |
| Severe dysarthria with near to unintelligible or worse | 2 | |
| Total score: | _____ | |
| Score interpretation | No stroke | 0 |
| Minor stroke | 1–4 | |
| Moderate stroke | 5–15 | |
| Moderate/severe stroke | 15–20 | |
| Severe (major) stroke | 21–42 | |
For hemorrhagic strokes, the Glasgow Coma Scale (GCS) neurologic assessment tool is used to describe the general level of consciousness in patients with traumatic brain injury or suspected hemorrhage stroke. Like the NIHSS, the GCS is not a diagnostic tool, and it does not replace the neurologic exam.
The Glasgow Coma Scale (GCS) is the gold standard used to objectively describe the extent of impaired consciousness in all types of acute medical and trauma patients. The scale assesses patients according to three aspects of responsiveness: eye-opening, motor response, and verbal response. Reporting each of these separately provides a clear, communicable picture of a patient’s state.
| Neurologic Aspect | Scale | Score |
|---|---|---|
| (Jain & Iverson, 2021) | ||
| Eye opening | Spontaneously | 4 |
| To sound | 3 | |
| To pain | 2 | |
| No eye opening | 1 | |
| Motor response | Obeys command | 6 |
| Localizes pain | 5 | |
| Withdraws from pain | 4 | |
| Abnormal flexion (decorticate) to pain | 3 | |
| Abnormal extension (decerebrate) to pain | 2 | |
| No motor response | 1 | |
| Verbal response | Oriented and converses | 5 |
| Disoriented and confused | 4 | |
| Inappropriate words | 3 | |
| Incomprehensible sounds | 2 | |
| No verbal response | 1 | |
| Total score: | ____ | |
| Score interpretation | Minor brain injury | 13–15 |
| Moderate brain injury | 9–12 | |
| Severe brain injury (comatose) | 3–8 | |
The highest score (15) indicates that the patient is fully conscious, and the lowest possible score (3) indicates the patient is comatose or deceased (Gaines, 2022).
Hypothesis and Diagnosis for Stroke Type and Etiology
As information accumulates, the stroke team builds evidence for the diagnosis of “stroke” or “nonstroke.” For likely strokes, the team will also be weighing the evidence for and against intracranial bleeding.
FORMING THE HYPOTHESIS
Hypothesis generation begins as soon as the first information about the patient becomes available, and as information is gathered, the clinician proceeds systematically from the more general to the more specific.
After a history is obtained, the clinician plans the physical examination to look for additional findings to help confirm or refute the preliminary diagnoses. Overall, the process of diagnosis should be logical, systematic, and sequential.
When physical examination is being done, it is important to consider the differential diagnoses that can mimic stroke. It has been reported that 19% of patients diagnosed with AIS by neurologists before cranial imaging actually had noncerebrovascular causes for their symptoms.
COMMON STROKE MIMICS
The most frequent stroke mimics include:
- Seizure
- Systemic infection
- Brain tumor
- Toxic-metabolic disorders such as hyponatremia and hypoglycemia
- Positional vertigo
- Conversion disorder (a psychiatric disorder in which the patient develops paralysis, numbness, blindness, deafness, or seizures, with no underlying neurologic pathology)
(Jauch, 2022)
After completing the initial assessment, the goal of subsequent evaluation is to determine the underlying pathophysiology of the stroke in order to guide therapy. After hemorrhagic stroke has been ruled out, the next stage of evaluation is focused on distinguishing between embolic and thrombotic stroke.
Features of the clinical history can be helpful in the determination of type of stroke, including:
- Clinical course: The pace and course of signs and symptoms and their clearing are the most important historical information for differentiating stroke subtypes.
- Ecology: The known demographic and historical features (including age, sex, and race) provide probabilities of having one or more of the stroke subtypes.
- Previous transient ischemic attack (TIA): A history of TIA, especially more than one, in the same territory strongly favors presence of thrombosis; attacks in more than one vascular territory suggest brain embolism from the heart or aorta.
- Activity at onset or just before the stroke: Hemorrhages can be precipitated by sexual activity or other physical activity, while thrombotic strokes are unusual under these circumstances. Sudden coughing and sneezing can precipitate brain embolism. Getting up during the night to urinate appears to promote brain embolism.
- Associated symptoms: The presence of certain associated symptoms can be suggestive of a stroke subtype. Examples include fever suggestive of embolic stroke due to endocarditis; infections that predispose to thrombosis; and accompanying symptoms such as headache, vomiting, seizures, and decreased level of consciousness.
(Caplan, 2022b)
CRANIAL IMAGING TO CONFIRM DIAGNOSIS
Because time is of primary importance, there should be a standing order for a cranial scan for all potential stroke patients. There should also be a plan for getting the scan read quickly. Cranial imaging should be completed within 25 minutes of the patient’s arrival at the ED, and the interpretation by the radiologist on call should be available within 20 minutes of the scan’s completion.
Imaging Studies
Imaging is done to exclude hemorrhage, assess degree of brain injury, and identify the lesion responsible for the ischemic deficit.
Noncontrast computed tomography (NCCT) of the head remains the mainstay in the setting of acute stroke. It is the most rapid and cost-effective strategy available. Its main limitation, however, is limited sensitivity in the acute setting. The goals of CT in the acute setting are to exclude intracranial hemorrhage, look for early features of ischemic stroke, and exclude other intracranial pathologies that may mimic a stroke.
Multimodal CT techniques, including CT perfusion imaging and CT angiography, make CT capable of addressing all acute imaging needs:
- Ruling out thrombectomy candidates
- Ruling out hemorrhage
- Identifying large vessel occlusion
- Detecting infarct core and penumbra
- Assessing collateral flow
CT perfusion has become a critical tool in the selection of patients for thrombolytic treatment as well as increasing the accurate diagnosis of ischemic stroke by nonexpert readers fourfold compared to routine noncontrast CT. It allows both the core of the infarct to be identified as well as the surrounding penumbra that can potentially be salvaged.
CT angiography may identify thrombus within an intracranial vessel and may guide intra-arterial thrombolysis or clot retrieval. It may also establish stroke etiology and evaluate carotid and vertebral arteries in the neck.
Multiphase or delayed CT angiography is showing benefit, either replacing CT perfusion or as an additional fourth step in the stroke CT protocol, as it guides patient selection for endovascular therapy by assessing collateral blood flow in the ischemic and infarct tissue.
MRI with magnetic resonance angiography (MRA) has been a major advance in the neuroimaging of stroke. MRI not only provides great structural detail but also can demonstrate early cerebral edema. In addition, MRI has proven to be sensitive for detection of acute intracranial hemorrhage. However, MRI is not as available as CT scanning in emergencies, many patients have contraindications to MRI imaging (e.g., pacemakers, implants), and interpretation of MRI scans may be more difficult.
Diffusion-weighted imaging (DWI) is highly sensitive to early cellular edema, which correlates well with the presence of cerebral ischemia. DWI shows far greater contrast and is superior at highlighting tissue injury within minutes of a cerebral infarct. It provides information on the viability of brain tissue, showing image contrast that is dependent on the molecular motion of water, which may be substantially altered by disease.
Transcranial doppler ultrasound (TCD) is useful for evaluating more proximal vascular anatomy—including the middle cerebral, intracranial carotid, and vertebrobasilar artery—through the infratemporal fossa. It has been utilized for the diagnosis of intracranial vessel occlusion, as well as for the differentiation between ischemic and hemorrhagic stroke, in the context of a negative CT and a clinically suspiscious patient presentation.
The use of single-photon CT (SPECT) scanning in stroke is still experimental and available only at select institutions. Theoretically, it can define areas of altered regional blood flow.
(Sharma, 2022; Jauch, 2022)
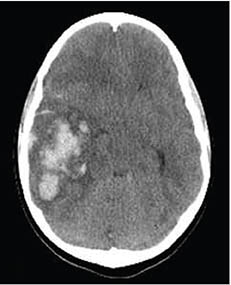
CT scan showing a hemorrhagic stroke (lighter-shaded area on the left). (Source: James Heilman.)
LUMBAR PUNCTURE (LP)
A lumbar puncture is done following a negative noncontrast CT if the history and symptoms contribute to increased clinical suspicion for subarachnoid hemorrhage. A lumbar puncture can be performed up to 12 hours from onset of symptoms.
The lumbar puncture involves the collection of at least four tubes of cerebrospinal fluid (CSF) to detect xanthochromia, a pink or yellow coloration of the CSF caused by the breakdown of red blood cells and subsequent release of heme pigments, including bilirubin This is sometimes the only sign of an acute subarachnoid hemorrhage. Xanthochromia is typically present in the CSF within 6 to 12 hours after the onset of symptoms (Patel et al., 2021).
OTHER DIAGNOSTIC PROCEDURES
Once a determination of the cause of a stroke is made, other diagnostic procedures may be required to aid in decision-making for treatment.
Carotid ultrasound is a two-step procedure that uses sound waves to create detailed images of the buildup of plaque and spectral analysis to measure blood flow velocity in the carotid arteries. It is done if the patient has a TIA or a medical condition that increases the risk of stroke.
Cerebral angiogram uses a catheter under X-ray imaging guidance and injection of contrast material to examine blood vessels in the brain for abnormalities such as aneurysms, plaque buildup, or thrombosis. The procedure produces very detailed, clear, and accurate images of blood vessels in the brain (Mayo Clinic, 2022).
Transesophageal echocardiogram is usually recommended when a very specific part of the heart requires imaging with greater resolution. It allows for better visualization and definition of structures within the heart and can be especially helpful in identifying thrombi (Stanford Medicine, 2021).