WOUND INFECTION
Open wounds present an unrestricted environment for organisms to live and reproduce. Infections always obstruct wound healing, and infection is the most common impediment to wound healing.
Bacteria are present on the surface of all open wounds (which is referred to as colonization), and this low level of contamination does not need to be treated. However, wounds that have been contaminated with significant numbers of bacteria will most likely result in infection. The degree of infection is dependent on several factors, including the type or types of microorganisms present and how virulent they are.
Within hours of an injury, neutrophils and macrophages migrate into the wound and begin removing debris. Large amounts of bacteria, however, cannot be removed within the normal reaction phase. When contamination persists, the influx of white blood cells continues, too. But these neutrophils die after 24 hours, and when they are continuing to infiltrate the wound because of persistent contamination, the dead neutrophils pile up and begin to clog the wound in the form of pus. Pus slows the formation of granulation tissue and the re-epithelialization of the wound, giving bacteria still more time to multiply. Furthermore, many bacteria secrete toxins that add to the tissue damage in the wound when it has become infected.
Types of Infectious Organisms
Bacteria exist in several different shapes and sizes: round, cylinder, and rods. The most widely known classification of bacteria is as either gram positive or gram negative, determined by a staining technique first developed by the Danish scientist Hans Christian Gram in 1884. Gram staining is still widely used today to quickly distinguish between gram-positive organisms (including species of Enterococcus, Staphylococcus, and Streptococcus) and gram-negative organisms (including Pseudomonas, Escherichia coli, and Klebsiella).
Another classification of bacteria important to the clinician is aerobic vs. anaerobic. Aerobic organisms live and thrive in an oxygen-rich environment, whereas anaerobic organisms do not need oxygen to survive and in some instances will die when exposed to it. Anaerobic organisms are a particular threat in wounds with areas of deep tunneling. In any wound where there is a likelihood of anaerobic organisms, a cover dressing that is impervious to oxygen should not be used. Identification of such organisms is made on tissue biopsy or through the use of an anaerobic culture kit.
Free-floating (planktonic) bacteria are what clinicians are most familiar with, and these organisms are most easily cleared from the wound. In many cases they can be eradicated by the patient’s own immune system or the introduction of antimicrobials into the wound treatment. In more severe cases, antibiotic therapy may be required (Bryant & Nix, 2016; Baranoski & Ayello, 2020).
BIOFILM AND INFECTION
One of the most persistent and difficult-to-treat forms of wound infection is biofilm formation (see above under “The Healing Process and Chronic Wounds”). Biofilms are collections of organisms comprised of bacteria, fungi, and other microorganisms existing together in a synergistic community, enveloped in a polymeric matrix that attaches tightly to the wound surface. Biofilms are more prevalent in chronic wounds (3 in 5) than in acute wounds (1 in 12) (Baranoski & Ayello, 2020).
The organisms in a biofilm have the capacity to decrease their metabolism, which in turn decreases their need for oxygen and nutrients, permitting them to become dormant and to reactivate later. Biofilm formation will increase the level of wound inflammation and also support the production of anaerobic organisms in the wound. Biofilms can negatively impact the wound healing process without exhibiting the normal symptoms of infection.
Research shows that biofilms develop in three phases:
- Biofilms form from a small group of bacteria and other microorganisms that have fastened themselves to the wound surface. This early phase of development provides clinicians with the best opportunity to halt biofilm development by cleaning and debriding the wound.
- The biofilm forms a stronger attachment to the wound bed, creating an organized symbiotic community.
- The biofilm produces an extracellular viscous material that forms a protective barrier for the biofilm. At this stage the biofilm may be detected as a tacky, shiny film on the wound surface.
From beginning to end, the process of biofilm formation happens over 2–4 days (Wound Source, 2018).
Biofilms can proliferate 2 mm below the wound surface and infiltrate surrounding healthy tissue (Shah et al., 2018; WOCN, 2022). Currently, there are no tests available to clinicians to detect biofilms in wounds.
Effective treatment for biofilm eradication is weekly sharp debridement of the wound followed by the immediate application of a broad-spectrum antimicrobial agent to prevent organisms freed during debridement from creating a new biofilm. Cadexomer iodine and silver agents have been shown to be successful in treating biofilms when used as a second step in treatment along with debridement.
Research indicates that biofilms can reform in wounds as quickly as 24 hours after debridement. The recommendation is to perform serial debridements no more frequently than once every seven days (Wound Source, 2018; Baranoski & Ayello, 2020).
Signs and Symptoms of Infection
The classic signs of wound infection include:
- Fever
- Pus
- Abscess
- Abnormal smell (malodor)
- Cellulitis
- Persistent inflammation with an exudate
- Warmth and redness
- Delayed healing
- Continued or increasing pain
- Edema
- Weak, crumbly granulation tissue that bleeds easily
If any of the above signs of wound infections are present, a wound culture is obtained to determine the appropriate course of treatment.
Infection is usually easily detected in acute wounds, and many of the above symptoms will be recognizable. But in chronic wounds, infection can be difficult to detect, and the presenting symptoms may be subtle. For example, the first indication of infection in a diabetic foot ulcer is oftentimes unexplained elevated blood sugars. The patient may complain that “for no reason my fasting blood sugar is running over 200 mg/dL every morning.”
Increased wound drainage can be another symptom of infection in a chronic wound. If a certain amount of redness has always been present in the periwound area, cellulitis may not be detected. The clinician must look closely for changes in the quality of the granulation tissue that appear “liver-like,” which is evidenced by a dark red color and more friable tissue (falls apart easily). It is important to keep in mind that the majority of patients with these signs will not run an elevated temperature, and so a normal temperature reading cannot be taken as a sign that the patient is infection free.
In chronic wounds in which bone is exposed or the clinician can probe down to bone, there is a high probability that tissue and bone infection (osteomyelitis) are present.
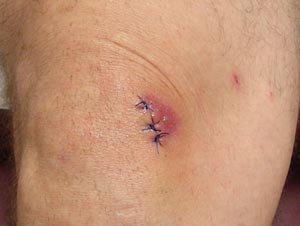
The redness around this sutured laceration (on the knee) may indicate a normally healing wound still in the inflammatory phase of healing, or it may signal the beginning of a wound infection. (Source: Antonio M. de Gordon, MD.)
ANSWERING PATIENT QUESTIONS
Q:How do I know if a wound is infected?
A:Seek medical attention if you have any of these signs and symptoms of an infection:
- Fever
- Pus in the wound
- Increasing redness of the wound or the area around it
- Swelling
- Persistent or increasing pain
- Redness or red streaks spreading out from the wound
Diagnosing Infection
Wound cultures are most commonly used to confirm the presence of infection. They include:
- Swab culture
- Tissue culture
- Aspiration culture
- Bone culture
It is important to remember that necrotic tissue and slough should not be collected as part of a wound culture.
SWAB CULTURE
Swab culture is one of the most frequently used methods to collect wound cultures, but it is not the most reliable. The surface of all chronic wounds is contaminated, and this will be evident in the culture. But this may not give a true picture of what is happening at a deeper level in the wound tissue.
The technique used to collect the culture is extremely important. First, the wound bed is thoroughly cleaned with sterile normal saline. Next, using an area approximately 1 cm2, the culture swab is pressed against the wound surface with sufficient pressure to produce wound exudate. The swab is then rotated to capture this exudate.
TISSUE CULTURE
Tissue culture, also referred to as punch biopsy, is considered the “gold standard” for accurately collecting wound culture. One disadvantage of this procedure is that a punch biopsy can only be performed by a physician, nurse practitioner, or physician assistant, which limits the settings in which it can be obtained. Punch biopsies are performed using sterile technique. Injectable, local anesthesia is administered to numb the area, which is then cleansed with sterile normal saline. Using a 3 mm curette and sterile scissors, the clinician removes a tissue sample from the wound bed, which is then placed in a sterile container and transported to the laboratory.
ASPIRATION CULTURE
This technique is often used to collect fluid from an abscess. It is done under sterile technique with local anesthesia. The procedure is performed by a physician, nurse practitioner, or physician assistant using a 10 ml syringe with a 22-gauge needle. The needle is inserted into the tissue adjacent to the wound and fluid is withdrawn. After the needle is removed from the area, the syringe is capped and sent to the lab for analysis.
BONE CULTURE
If bone is visible in the wound, the physician may elect to cut away a sample of the bone and send it for culture. This is a sterile technique, but it does not require local anesthesia because only bone fragments are being removed.
If a bone biopsy is not possible but osteomyelitis is suspected, then an X-ray or MRI of the affected area will be necessary. Although some insurance carriers may insist that an X-ray be used for diagnosis, clinicians must consider that an X-ray is not the most reliable means of confirming the presence of osteomyelitis and that an MRI may still be required.
Treating Infection
Prompt recognition of wound infection and early intervention can help prevent microorganisms from spreading to deeper tissues and possibly bone, with the risk of systemic infection. Topical antibiotics are not as widely used in wound care as they once were due to the increase in bacteria-resistant organisms and the possibility of hypersensitivity reactions (Baranoski & Ayello, 2020). For critically colonized wounds, antimicrobial dressings are the first choice of treatment.
The frequency of dressing changes may need to be increased during periods of infection to carefully monitor the wound and to address an increase in wound drainage that usually accompanies wound infection.
Where systemic infections are present, a specialist may be required. Such infections must be addressed promptly in order to prevent adverse consequences, such as the risk of limb amputation for a diabetic patient. Once culture results are available, appropriate antibiotic therapy will be ordered. Mild infections are usually treated with oral antibiotics. However, more severe infections such as osteomyelitis require intravenous antibiotics, and surgical removal of the infected bone may be done in conjunction with antibiotic therapy (WOCN, 2022).
Some wounds that have been sutured closed over extensive subcutaneous tissue dissection and debridement can develop a temporary inflammatory reaction in which they become red and edematous although they are not infected. If a clinician suspects this problem, the physician is contacted and may order that one or two stitches or staples be removed to lessen the tension. Any fluids in the wound are then gently expressed or aspirated, followed by packing the area with sterile saline-moistened gauze and then covering it with the appropriate dressing. In this case, the wound is cleansed daily and dressings packed/applied per physician instructions. This type of inflammatory reaction will decrease within 48 hours.
Preventing Infection
Preventing infection, environmental contamination, and cross infection are the responsibility of all clinicians involved in wound care. Any break in the skin surface provides a portal of entry for bacteria and other microorganisms into the body, and clinicians must be cognizant of this when performing dressing changes regardless of the location—facility, clinic, or home. In the home setting, the clinician is also responsible for educating caregivers about the means of preventing infection.
Patients with acute and chronic wounds are at particular risk for healthcare-associated infections, which remain a major threat to patient safety. The Centers for Disease Control and Prevention (CDC, 2018) reports that on any given day, 1 in 31 hospitalized patients has at a minimum one healthcare-associated infection (HAI). The key to elimination of HAIs is full adherence to recommendations across the continuum of care.
Preventing the spread of infectious organisms includes the following actions:
HAND HYGIENE
Since most infections occur with direct patient contact, proper hand hygiene (handwashing or using alcohol-based rubs) remains the single most effective way to prevent infection to and from patients. In order to prevent infection:
- Wash hands or use an alcohol-based product immediately after gloves are removed, between patient contacts, and when otherwise indicated.
- Wash hands between tasks and procedures on the same patient to prevent cross-contamination of different body sites.
- Avoid unnecessary touching of surfaces near the patient to prevent contaminating clean hands and to prevent transmission of pathogens from contaminated hands to surfaces.
- Do not wear artificial fingernails or extenders.
STANDARD PRECAUTIONS
Standard Precautions are used with every patient. The degree of Standard Precautions implemented will be determined by the complexity of care. For interactions such as changing a dry dressing covering an intact surgical wound, only gloves may be needed. During other interactions (e.g., wound cleansing, irrigation, and wound debridement), use of gloves, gown, face shield, or mask and goggles may be required, and these should be readily available.
BARRIERS AND PPE
Personal protective equipment (PPE) is specialized clothing and/or equipment worn by a healthcare worker for protection against a hazard. PPE provides barriers to the transmission of infectious organisms during wound care, thereby protecting both the healthcare professional and the patient.
Types of PPE include:
- Gloves, both sterile and nonsterile
- Gowns of varying permeability
- Face shields
- Goggles
- Masks
- Head coverings
- Booties
Clinicians must follow these rules when using PPE:
- Know how to use the equipment.
- Always wear PPE in exposure situations.
- Remove and replace PPE that is torn, punctured, or has lost its ability to function.
- Remove clothing that becomes contaminated with blood or other potentially infectious material as soon as possible.
- Remove PPE before leaving the work area.
- Handle contaminated laundry as little as possible.
- Place contaminated PPE in appropriately labeled bags or containers until disposed of, decontaminated, or laundered.
- Know where these bags or containers are located in the work area.
(APIC, 2022)
ANSWERING PATIENT QUESTIONS
Q:Do I need to wear gloves when doing wound care at home?
A:It is better to wear gloves when doing wound care to decrease the risk of infecting the wound, but sterile gloves are not necessary. Gloves should be discarded after each dressing change.
Documentation
Clear, concise, and accurate documentation is an essential element of each wound care contact in all settings. Documentation allows all those involved in patient care to know the wound status and provides for good communication among all clinicians. It is also of paramount importance for reimbursement and in the case of litigation.
Documentation of the care given includes the following components:
- Date and time
- Interventions performed
- Wound characteristics, including the amount and type of drainage
- Wound odor
- Patient’s pain level during and after the treatment
- Interventions to relieve pain and the effectiveness of the interventions
- Patient’s level of anxiety before, during, and after treatment
- Patient’s reported level of comfort with applied dressings
- Supplies used
- Name and credentials of the clinician providing the care
Only approved abbreviations should be used and objective findings accurately described.