LONG-TERM TREATMENT OF COPD
COPD is a life-long disease. It requires special medical treatment during acute exacerbations, and after the disease reaches the “moderate” level, it requires daily medications and permanent adjustments to a patient’s lifestyle. GOLD guidelines offer a comprehensive framework for the management of COPD (GOLD, 2021).
The goals of long-term COPD treatments are to:
- Slow the progression of the disease
- Ease the symptoms
- Increase the patient’s ability to be mobile and carry out activities of daily living
- Prevent acute exacerbations
Education is important to improve quality of life and reduce hospital admissions. All patients with COPD should learn about their disease and understand that smoking and air pollution will further damage their lungs. Patients are instructed to make a special effort to avoid respiratory infections and to get yearly influenza vaccinations. In addition to yearly influenza vaccinations, it is recommended that all adults obtain a pneumonia vaccination after reaching age 65. Those at higher risk for pneumonia, such as patients with COPD, are urgently recommended to be vaccinated, often earlier than age 65 (GOLD, 2021).
ANSWERING PATIENT QUESTIONS
Q:What can be done for my COPD?
A:Treatment for COPD helps prevent complications, prolong life, and improve a person’s quality of life. Quitting smoking, staying away from people who are smoking, and avoiding exposure to other lung irritants are the most important ways to reduce your risk of developing COPD or to slow the progress of the disease if you have it.
Treatment for COPD includes medicines such as bronchodilators or steroids. Preventive therapies include flu and pneumococcal vaccines to avoid or to reduce further complications.
As the symptoms of COPD get worse over time, a person may have more difficulty walking and exercising. You should talk to your primary care provider about exercise programs. Ask whether you will benefit from a pulmonary rehab program—a coordinated program of exercise, physical therapy, disease management training, advice on diet, and counseling.
Oxygen treatment and surgery (to remove part of a lung or even to transplant a lung) may be recommended for patients with severe COPD.
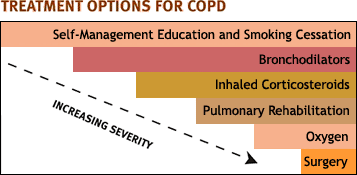
At each stage of the disease, there are characteristic medical therapies:
- Mild COPD (GOLD 1) is usually treated with short-acting bronchodilators, which are used as needed for dyspnea.
- Moderate COPD (GOLD 2) requires regular treatments with bronchodilators, sometimes with the addition of inhaled corticosteroids. At this stage, patients are often enrolled in a pulmonary rehabilitation program.
- Severe COPD (GOLD 3) typically requires two or more bronchodilators regularly. Inhaled corticosteroids are added to the regimen to prevent repeated acute exacerbations.
- Very severe COPD (GOLD 4) usually requires the addition of long-term oxygen therapy. Surgical treatments can be appropriate at this stage.
(Source: National Institutes of Health.)
Therapeutic Lifestyle Changes
Medications are the fundamental day-to-day tools for controlling the symptoms of COPD, but there are also effective nonpharmaceutical techniques for treating COPD. These include patient education, smoking cessation, keeping airways clear, and nutritional therapy.
PATIENT EDUCATION / ENERGY CONSERVATION
Self-management interventions attempt to motivate, engage, and coach COPD patients to adopt positive health behaviors and manage their disease better on a day-to-day basis by learning effective skills. Patient education for COPD patients includes:
- Risk factors
- Signs and symptoms of exacerbations
- Compliance with treatment
- Maintaining contact with healthcare providers
- Addressing the complex psychosocial factors of having COPD
(GOLD, 2021)
COPD patients who use the education given to them to establish healthier self-management behaviors may experience significantly fewer hospital readmissions in the short run (GOLD, 2021).
Following are guidelines a clinician can follow in the area of patient education:
- Teach patients with COPD about their disease. Explain that the disease causes irreversible and progressive problems. Warn patients that they will have episodes in which the symptoms—difficulty breathing, wheezing, productive cough, and tiredness—get worse for days or even weeks.
- Assure patients that they will be helped by medications that make breathing easier. Tell them there are several things they can do to slow the progression of the disease and to lessen the number of acute exacerbations. The most crucial step is to stop smoking. Although smoking has already damaged their lungs, continued smoking will increase the damage and will make their COPD worsen more quickly.
- Let patients with COPD know that they should make every effort to stay active while recognizing the need to monitor and time their efforts throughout the day. In addition, give them practical suggestions that will help them to cope with the inevitable limitations posed by COPD (see box below).
ENERGY CONSERVATION TIPS
- Simplify your tasks and set realistic goals. Do not think you have to do things the same way you have always done them.
- Plan your activities (chores, exercise, and recreation) ahead of time. Space out your activities throughout the day. Do not schedule too many things to do in one day. Do the things that take more energy when you are feeling your best.
- If needed, rest before and after activities.
- If you become tired during an activity, stop and rest. You might need to finish it on another day or when you feel less tired.
- Do not plan activities right after a meal. Rest 20–30 minutes after each meal.
- Ask for help. Divide tasks among family and friends.
- Get a good night’s sleep and elevate your head when sleeping. Be careful not to nap too much during the day or you might not be able to sleep at night.
- Do all of your grooming (shaving, drying your hair, etc.) while sitting.
- If needed, use devices and tools that assist you, such as a walker, shower chair, hand-held shower head, bedside commode, or long-handled tools for dressing (such as a dressing stick, shoehorn, or sock donner).
- Wear clothes that have zippers and buttons in the front so you do not have to reach behind you.
- If your doctor says it is okay, you may climb steps. You might need to rest part of the way if you become tired. Try to arrange your activities so you do not have to climb up and down stairs many times during the day.
- Avoid extreme physical activity. Do not push, pull, or lift heavy objects (more than 10 pounds) that require you to strain.
(Cleveland Clinic Foundation, 2018a)
SMOKING CESSATION
The cornerstone of management of COPD is smoking cessation.
Nearly 40 million people in the United States smoke, including 4.7 million middle and high school students who currently use some form of tobacco, including e-cigarettes. Every day 1,600 people in the United States under 18 years of age smoke their first cigarette (CDC, 2021e). Most patients with COPD have a long smoking history, and many will still be smoking when they are under medical care. From day one, clinicians should strongly urge patients to stop smoking.
Quitting can be difficult since the nicotine in tobacco smoke is powerfully addictive. In addition, the rituals of smoking fill basic psychological needs for many individuals. Therefore, when healthcare professionals merely tell patients to stop smoking, their patients succeed over the long term only 5% of the time. On the other hand, a large study of a smoking cessation program that combines nicotine replacement therapy Wabbr medications with cognitive behavioral therapy (CBT) showed a 32.8% successful quitting rate, and another study of NRT alone showed a 22.2% success rate after three months (Aksel et al., 2021; Arslan et al., 2021).
Healthcare professionals are vital to the success of a smoking cessation program. They may begin by saying to patients: “COPD cannot be cured, but if you continue smoking, the disease will worsen much more quickly. Have you thought about quitting smoking?” Regardless of the patient’s answer, they follow with the offer, “When you’re ready to stop smoking, I’ll be happy to collaborate with you to set up as effective a program as possible.”
Successful smoking intervention programs begin by asking the patient to set a specific quitting date. The programs then maintain continued contact with the patient to provide medication, counseling, support, advice, and a modicum of social pressure. (See also “Resources” at the end of this course.)
The “Five As” for Counseling Smokers
Clinicians can use the Five As when counseling their patients who smoke. Taking even one step is constructive.
- Ask about the tobacco use and identify and document tobacco use status of every patient at every visit.
- Advise the patient to quit and provide information on the benefits of quitting.
- Assess whether the tobacco user is willing to quit at this time. Are there any challenges to remaining abstinent?
- Assist the patient with finding resources and coming up with a cessation plan. Offer medication and provide or refer for counseling or additional behavioral treatment to help the patient quit. For patients unwilling to quit at this time, provide motivational interventions designed to increase future quit attempts. For the recent quitter and anyone with remaining challenges, provide relapse prevention.
- Arrange follow up to help the patient follow through with quitting.
(AHRQ, 2019)
ANSWERING PATIENT QUESTIONS
Q:Why should I quit smoking?
A:Statistically, people who stop smoking live longer. If you quit smoking before you are 35, you will live about six years longer. Even if you quit at age 55, you may still add two years to your life. By quitting smoking, you reduce your chances of getting lung disease, heart disease, and cancer. You will feel better and healthier. Smoking injures your senses of taste and smell, and quitting smoking will even make food taste better.
Q:I like to smoke, and I know people who have lived a long time even though they were smokers. Why should I go through the agony of stopping something I enjoy? Besides, I may not even be able to quit.
A:Cigarettes are legal addictive drugs, and they are easier to buy and less expensive than illegal drugs—but smoking is gambling, with bad odds. As a smoker, you have a 1-in-3 chance of dying earlier than you would if you quit. When you do die, it will most likely be of heart disease, stroke, cancer, or COPD. Smoking is responsible for about 1 out of every 5 deaths in the United States, and almost a half million Americans die each year from diseases caused by smoking.
Your smoking can also hurt the people around you. Breathing in another person’s smoke can cause lung problems in children and cancer and heart disease in adults. Pregnant women and new mothers and fathers can protect their baby’s health by stopping smoking now.
Sure, it is tough to quit smoking. Staying healthy and protecting the health of the people around you is difficult. But it’s important not to hide behind the excuse that you won’t be able to stop smoking. Studies suggest that most people can quit smoking with the combination of behavioral and pharmacologic therapy.
Q:What is the first thing I need to do once I’ve decided I want to quit?
A:You should set a quit date. Then make an appointment to see your primary care provider before the quit date. They will help you devise a plan that will make quitting easier.
Also, plan to join a support group or a stop-smoking program. The American Lung Association has an online stop-smoking program called “Freedom from Smoking Online.” Another helpful organization is Nicotine Anonymous, which runs 12-step programs with group support. (See also “Resources” at the end of this course.)
Here are some other general tips:
- Pick a suitable time to quit, a time when you won’t be under a lot of stress.
- Face the fact that it may not be easy and that you may have uncomfortable symptoms for a few weeks. You may get headaches or be sleepy or dizzy. You may become irritable or nervous. You will probably have cravings for a cigarette.
- Add some extra exercise to your quitting program. Walking, for example, is a great stress reducer.
- Tell your friends and family you are trying to quit smoking. Get their help to distract you, to keep up your spirits, and to be there when you need to complain.
Pharmacologic Therapy for Smoking Cessation
The pharmacologic aspect of smoking cessation programs attempts to ease the effects of nicotine withdrawal. Smokers who need their first cigarette within a half-hour of getting up in the morning are likely to be highly addicted to nicotine. When these people stop smoking, they become anxious, irritable, easily angered, easily tired, and depressed. Their sleep is disrupted. They have difficulty concentrating. These withdrawal effects are common during the first 2–3 weeks after quitting.
FDA-approved medications for the treatment of tobacco use include:
- Nicotine gum
- Nicotine inhaler
- Nicotine lozenge
- Nicotine nasal spray
- Nicotine patch
- Bupropion SR (Wellbutrin, Zyban)
- Varenicline (Chantix)
(McCuistion et al., 2020)
Nicotine Replacement Therapy (NRT). To lessen withdrawal symptoms, nicotine can be taken in low doses without smoking to relieve the symptoms of craving. The transdermal patch is the only form of NRT that secretes a continuous dose of nicotine to prevent cravings. Other forms of nicotine replacement include gum, lozenges, inhalers, and nasal sprays; these should be used on an as needed (PRN) basis for cigarette cravings for about two weeks, and then the doses should be tapered. The gum, lozenges, and inhaler help to satisfy oral cravings, and the inhaler raises nicotine blood levels more rapidly than the other routes of administration.
Nicotine patches are marketed as Habitrol and NicoDerm CQ; nicotine gum includes Nicorette. Prescription nicotine replacement therapy is available only under the brand name Nicotrol, available both as a nasal spray and an oral inhaler.
Nicotine replacement products can cause the following side effects:
- Nausea
- Dizziness
- Weakness, vomiting, fast or irregular heartbeat, mouth problems (with the lozenge or gum)
- Redness or swelling of the skin around the patch that does not go away when the patch is removed
If these side effects occur, the patient is instructed to discontinue the product’s use and report the effects to their primary care provider (McCuistion et al., 2020).
Since nicotine is a vasoconstrictor, people with coronary artery disease (CAD) are advised not to use any nicotine replacement therapy. Vasoconstriction causes increased arterial resistance that may result in an increase in blood pressure. Additionally, individuals with CAD with increased vasoconstriction may experience a reduction in the diameter of the coronary arteries. This can lead to angina or myocardial infarction. In the hospital setting, patients with CAD who are experiencing nicotine withdrawal are given sedatives to reduce symptoms rather than nicotine replacement medications for these reasons.
Antidepressants. The FDA has approved the antidepressant bupropion SR (sustained-release) to help patients for whom nicotine replacement therapy has not worked. Bupropion raises levels of dopamine in the brain, which helps to relieve nicotine cravings. Treatment usually lasts for 7–12 weeks but may be continued for the needed therapeutic effect. It should not be stopped abruptly; patient’s should be weaned off slowly to avoid withdrawal symptoms.
Nicotine agonists. The FDA has approved varenicline (Chantix), a nicotine agonist, for anti-smoking therapy. Varenicline binds to nicotine receptors and prevents nicotine from activating the receptors, while producing a smaller stimulant effect than nicotine. Since varenicline contains no nicotine, it does not cause vasoconstriction that can reduce blood flow to the myocardium, making it the drug of choice for patients with a cardiac history. It also stimulates the release of dopamine. Side effects may include nausea and vomiting, insomnia, headache, abnormal dreams, constipation, diarrhea, fatigue, malaise, upper respiratory tract infection, dyspnea, chest pain, abdominal pain, xerostomia, appetite changes, rash, or emotional disturbances.
Electronic or e-cigarettes contain vaporized liquid nicotine and are believed to aid in smoking cessation by reducing the amount of nicotine used, but there is not yet sufficient research to support this. No prescription is needed.
ANSWERING PATIENT QUESTIONS
Q:What medicines should I take when I’m trying to stop smoking?
A:There are a variety of antismoking medicines, and your primary care provider can suggest the best one for you. Nicotine is an addictive drug. For many people, nicotine replacements help to keep withdrawal symptoms to a minimum. Nicotine replacements come as patches, gums, lozenges, and an inhaler. Your primary care provider can also prescribe a nicotine-free tablet called Chantix, which reduces withdrawal symptoms. Some people get help from the antidepressant bupropion, which is a prescription medicine.
Q:Aren’t nicotine replacement products just as bad as smoking?
A:No, nicotine replacements do not have all the tars and poisonous gases that are found in cigarettes. Furthermore, these medicines give you less nicotine than a smoker gets from cigarettes. Nicotine replacement products (patches, gums, lozenges, or inhalers) should not be used by people who are pregnant or nursing. People with other medical conditions should check with their primary care provider before using any nicotine replacement product. It is important that smokers quit smoking completely before starting to use nicotine replacements.
CASE
Elsa Hunter is a patient with moderate COPD who is struggling with quitting smoking. She has tried nicotine patches and gum, the nicotine agonist Chantix (varenicline), hypnotherapy, acupuncture, and counseling. Each method has been temporarily successful, and then Ms. Hunter has started smoking again. Ms. Hunter states that the patches caused skin irritation and scarring, the gum didn’t work, Chantix caused severe nausea, and the hypnotherapist “couldn’t put her under.”
Although Ms. Hunter understands the dangers of smoking and the effects on her health, she continues to return to smoking in times of stress. The nurse sits with Ms. Hunter to discuss the types of stressors that trigger her addiction and some strategies to avoid them or to manage them in other ways. The physician also orders the antidepressant Zyban (bupropion) to reduce nicotine cravings.
At her next follow-up visit, Ms. Hunter informs the nurse that she has been smoke-free for the past six months “thanks to the Zyban.”
KEEPING AIRWAYS CLEAR
Patients with COPD with significant chronic bronchitis must keep their airways clear. They should be encouraged to cough up sputum, and they should not get in the habit of using cough suppressants or sedatives. Postural drainage can help patients who cannot clear their secretions by coughing. This is a technique that patients can be taught to employ at home in which they place themselves in a variety of body positions that encourage gravity-assisted drainage of the lungs.
Most people’s lungs secrete extra mucus in response to inhaled irritants. To avoid stimulating excess secretions, patients with COPD are advised to stay out of smoke-filled rooms and to stay indoors during air pollution alerts. Home air conditioners and air filters are effective at keeping indoor air clear of particulates.
ANSWERING PATIENT QUESTIONS
Q:I have COPD. What can I do at home to keep the air clean?
A:It is important to keep the air in your home clean. Keep your windows closed and stay indoors when there is a lot of pollution or dust outdoors. When you cook, keep smoke and cooking vapors out of the air by using an exhaust fan or opening a door or a window. Don’t let anyone smoke in your house. Unless your fireplace is the only way for you to heat your home, you should not burn wood or kerosine in your home. Avoid using any aerosol (spray) products. Don’t use strong perfumes. When your house is being painted or sprayed for insects, stay away as long as you can or until the fumes have disappeared.
NUTRITIONAL THERAPY
The symptoms of COPD improve when patients who are overweight lose weight. Some patients with COPD, however, have the opposite problem: they have become thin and malnourished. Weight loss is a predictor of more exacerbations and a poorer prognosis.
Weight loss results in part from the high energy cost of breathing with COPD. In addition, the chronic inflammatory state underlying COPD tends to put the body’s metabolism into a catabolic state, in which larger molecules such as tissue are broken down into smaller molecules. This constant breakdown of tissue increases the body’s metabolic rate, causing further weight loss. Other factors that may contribute to malnutrition are poor appetite, altered taste due to mouth breathing, sputum, fatigue, anxiety, depression, infections, and side effects of medications (Harding et al., 2020).
To help maintain a healthy body weight, malnourished patients with COPD are given dietary counseling that includes specific recommendations for meals and meal supplements that are nutritionally balanced and that contain sufficient calories to make up for the work of breathing. Additional calories are also recommended to balance the calories burned during the exercise training recommended for people with COPD (GOLD, 2021).
Suggestions for decreasing dyspnea and conserving energy to lower the number of calories burned include:
- Rest and avoid nebulizer treatments, if possible, one hour before and after meals.
- Use a bronchodilator (inhaler) before meals.
- Use an oxygen nasal cannula during meals if oxygen is needed while eating.
- Have broken or missing teeth fixed to make eating easier.
- Walk or get out of bed for meals to promote appetite.
Patients can also be taught to increase calorie and protein intake without having to increase the amount of food they eat. An additional 25–45 kcal per kg of body weight and 1.5 grams of protein per kg of body weight may be needed to maintain body weight. The malnourished COPD patient may need up to 2.5 grams of protein per kg of body weight to restore muscle mass.
Suggestions for improving weight gain include:
- Eat higher-calorie foods first.
- Limit liquids at mealtimes so as not to fill up on them.
- Eat meals more frequently (five or six per day) and have snacks between meals.
- Add margarine, butter, sauces, mayonnaise, gravies, and peanut butter to food.
- Keep favorite foods and snacks available.
- Eat cold foods (which cause less of a feeling of fullness than hot foods).
- Keep prepared meals available for times when too fatigued or short of breath to prepare meals or snacks.
- Eat larger meals when not as tired.
- Avoid gassy foods (cabbage, beans, cauliflower).
- Add skim milk powder (2 tbsp) to regular milk for additional calories and protein.
- Use milk and half-and-half instead of water when making soups, puddings, and cocoa.
- Add grated cheeses to sauces, casseroles, soups, potatoes, sandwiches, and vegetables.
- Choose desserts that contain eggs (custards, sponge cake, angel food cakes, bread pudding, rice pudding, and smoothies).
(Harding et al., 2020)
Pulmonary Rehabilitation and Integrated Care
Pulmonary rehabilitation (PR) is the term for a comprehensive, evidence-based, multidisciplinary program designed to assist patients with COPD who are having difficulty with breathing and ADLs (activities of daily living). A self-management COPD program is structured but personalized and works on motivating and supporting patients to adopt positive health practices and develop skills to better manage their illness.
The PR process uses collaborations between COPD patients and healthcare personnel. Most PR programs involve a respiratory therapist, occupational therapist, physical therapist, and dietitian. Physicians, pharmacists, and nurses may also be involved, but not at every meeting with the patient. PR programs are delivered in inpatient, outpatient, clinic, physician office, telehealth, and home settings. A multidisciplinary and multifaceted pulmonary rehabilitation program has been shown to reduce the number of hospital readmissions for a three-month period for COPD patients (GOLD, 2021).
PR programs include assessment, exercise therapy, education, and psychological support. The benefits are maximization of functional status and the reduction of healthcare costs by promoting self-management of symptoms. Some rehabilitation programs continue for an extended time, but most run for a few weeks and then give patients individualized instructions for continuing at home.
The primary objective of a PR program is to restore individual patients to as independent a level of function as possible with an improved health-related quality of life. Evidence has shown that dyspnea symptoms improve in patients with COPD who undergo a PR regime. PR is proven to be a cost-effective treatment model and reduces the number of hospital admissions, but it cannot be substantiated that PR extends the life of patients with COPD (Harding et al., 2020).
EDUCATION IN PULMONARY REHABILITATION
Patient and family education are central to all PR programs. In these education sessions, patients and their families learn details about COPD and its treatment. Education informs the patient and family in how to self-manage the disease in collaboration with the various PR disciplines. Education topics may include understanding chronic lung disease, medications, breathing control, oxygen therapy, heart health, falls prevention, diagnostic tests, and advance care planning. Increasing patient knowledge leads to essential behavior changes, although education alone does not improve outcomes (GOLD, 2021).
ANSWERING PATIENT QUESTIONS
Q:What is pulmonary rehab?
A:Pulmonary rehabilitation is a program that includes regular exercise, training in how to manage your disease, and practical advice, all of which help you to stay active and remain able to conduct your day-to-day activities. After some medical breathing evaluations, you will meet with a pulmonary rehab team and make a plan that is best for your disease and your lifestyle. Usually, there are meetings, exercise classes, suggestions for long-term improvements in your lifestyle, and an advisor whom you can always contact for advice.
ASSESSMENT TESTING
COPD assessment testing measures several parameters (cough, phlegm, chest tightness, breathlessness, limited activities, confidence leaving home, sleeplessness, and energy) to determine a COPD patient’s degree of symptom severity and exacerbation risk. Several other assessment tests can be used to evaluate COPD patients for their ability to tolerate exercises and endurance training.
The 6-minute walk test (6MWT) is well-known endurance field test used to predict a COPD patient’s tolerance for endurance training through exercise (Zeng et al., 2018). Another widely used COPD patient assessment tool is the Modified British Medical Research Council Questionnaire (mMRC). This is a simple, five-question tool that measures the degree of breathlessness. It is also considered a predictor of future mortality risk (Moradkhani et al., 2021).
The most commonly used tests to assess balance in COPD and other chronically ill patients are the Berg Balance Scale (BBS) and the Balance Evaluation Systems Test (BEST). The BEST test can also be modified and administered as the Mini BEST test and the Brief BEST test for patients who are unable to tolerate the complete BEST test because of muscle weakness, pain, or dyspnea. These tests can also be used after six weeks of an intervention of balance training to assess whether or not the training has been effective (Abrantes et al., 2021; Lauha Chung & Kit Yi Mak, 2021).
PROGRAM PLANNING
PR programs are tailored to the needs of each individual. The number of sessions per week, need for nutritional counseling, need for psychological support, and need for oxygen supplementation are determined after a careful assessment of each patient’s capabilities and desired goals. The best possible benefits occur in a PR program lasting 6–8 weeks. There is no evidence of any additional benefits from expanding the program to 12 weeks’ duration. The type of exercise modalities are specifically individualized regarding the duration of each exercise, the number of repetitions, the number of weights, and the progression for increasing exercise (GOLD, 2021).
EXERCISE TRAINING
A goal of PR is to optimize the functional status of a patient with COPD by exercise training and collaborative self-management. Exercise training supervised by occupational and physical therapists does not improve lung functioning, but it can reduce COPD symptoms and increase the amount of exercise that the patients can do without being stopped by dyspnea. It can also reduce the number of hospitalizations for acute exacerbations.
Physical inactivity is the greatest source of the muscle weakness that affects patients with COPD, causing exercise intolerance and the wasting of skeletal and respiratory muscles. Although people with COPD have irreversible breathing difficulties, exercise training—including interval training, strength training, upper- and lower-limb training, and transcutaneous neuromuscular electrical stimulation—can significantly increase a patient’s strength and endurance and reduce their fatigability. These improvements result from increased muscle size (specifically, cross-sectional area), increased blood flow to muscles, increased oxidative enzyme capacity, and reduction of lactic acid production during exercise (Sánchez-Nieto et al., 2021).
Endurance training improves exercise-induced hyperinflation, exertional dyspnea, heart-rate recovery, and muscle dysfunction in COPD. Endurance training in the form of walking and cycling retards the progression of activity intolerance in patients with COPD, as do unsupported upper extremity exercises, such as cross-body weight-lifting.
Typical PR programs include graded aerobic exercises, such as regular sessions of walking or stationary bicycling three times weekly. The walking exercise program, for example, might begin with slow treadmill walking for only a few minutes. Gradually, the length and speed of the walking is increased. The goal would be for the patient to walk in gradually increased increments without needing to stop because of shortness of breath. At that point, the patient would be assigned a maintenance exercise program to be done at home, which may include stretching, weight training, and a stationary bicycle (Tounsi et al., 2021).
Strength training can improve not only muscle strength and quality of life but also exercise capacity in patients with COPD. Strength training utilizes either free weights (such as dumbbells) or weight machines. For a patient starting out with strength training, a repetition range of 8–12 is appropriate. The training frequency should be 2–3 days per week (Sá nchez-Nieto et al., 2021).
In one study using ankle weight cuffs during knee extension exercises in COPD patients hospitalized for acute exacerbations, subjects were able to increase their lower-extremity muscle strength and improve their Timed Up and Go (TUG) and Sit-to-Stand (STS) test scores (Tounsi et al., 2021).
Balance training can improve endurance and exercise capacity. COPD eventually causes skeletal muscle weakness, slow gait, and decreased physical activity ability secondary to dyspnea and fatigue from the overuse of respiratory muscles. Balance impairment is directly connected to falls, and research suggests that specifically improving balance control can result in significant improvement in the ability to perform ADLs more independently (Tounsi et al., 2021).
Supplemental oxygen is recommended during exercise for patients who experience severe exercise-induced hypoxemia. Oxygen saturation may need to be monitored in patients whose oxygen dependency may otherwise prevent them from exercising. Increased flow rates may enable oxygen-dependent patients to exercise longer and with less dyspnea. This may improve exercise endurance during a high-intensity exercise program.
BREATHING INSTRUCTION
Rehabilitation sessions also include breathing instruction that teaches patients how to slow their rate of breathing by pursing their lips and how to rest the upper respiratory muscles by using abdominal breathing instead of chest breathing.
PURSED-LIP BREATHING
Pursed-lip breathing improves breathing, releases air trapped in the lungs, expands the airways longer, and decreases effort. This breathing control exercise prolongs expiration to reduce the respiratory rate and improves breathing by maximizing inspiration. Pursed-lip breathing relieves shortness of breath and promotes general relaxation.
This technique can be used during the difficult part of any activity, such as bending, lifting, or stair climbing. It should be practiced 4–5 times a day to establish the correct breathing pattern. Pulmonary rehabilitation professionals can teach this technique by demonstrating the following steps:
- Relax the neck and shoulder muscles.
- Inhale slowly through the nose for two counts, keeping the mouth closed.
- Take normal breaths while counting “one, two.”
- Pucker or “purse” the lips as if whistling or gently flickering the flame of a candle.
- Exhale slowly and gently through pursed lips while counting “one, two, three, four.”
- Do not force the air out.
- Always breathe out for longer than breathing in.
- Breathe slowly and easily until in complete control.
(Cleveland Clinic Foundation, 2018b)
NEUROMUSCULAR ELECTRICAL STIMULATION (NMES)
Some patients with COPD have such poor lung function or such weak musculature that they cannot take part in the usual aerobic exercise training programs. Small studies suggest that transcutaneous electrical stimulation of the patients’ lower limbs can improve their muscle strength and exercise tolerance. This is an individualized protocol in which the intensity (amplitude), frequency, duration, and waveform of the stimulus is determined for each patient. Precise patient and family education are needed to ensure the device is properly used.
NMES increases muscle strength, exercise capacity, and standing balance, and reduces dyspnea of outpatients with severe COPD. Improvements may be seen in poor baseline exercise tolerance, and NMES can be used during acute COPD exacerbations. This has worked even for bedridden patients. Neuromuscular stimulation routines are safe for most patients, inexpensive, and can be done at home (Mekki et al., 2019).
PSYCHOLOGICAL SUPPORT
Multiple studies have proven there is a direct correlation between reduction of measurable anxiety and depression levels in patients with COPD when they participate in PR. Therefore, PR is recommended as a viable adjunct to other treatments for patients with COPD at any stage of the severity of the disease. The degree of psychological improvement brought about by the program warrants PR being included in treatment of anxiety and depression in this population (GOLD, 2021). Clinical depression as a comorbidity with COPD is associated especially with patients who are younger, female, and smokers, and with a lower FEV1, cough, history of cardiovascular disease, and poor prognosis.
ACTIVITIES OF DAILY LIVING (ADLs)
Occupational therapists (OTs) in particular play a key role in teaching patients with COPD to maintain their independence and abilities in performing necessary ADLs without developing activity intolerance. OTs are involved in approximately 30% of all individual pulmonary rehabilitation programs.
Screening tools for ADLs that may be used in PR settings include:
- Chronic Respiratory Disease Questionnaire-Dyspnea (CRQ-Dyspnea) is used to measure perceived dyspnea on a seven-point Likert scale in the problem or difficulty areas corresponding with the COPM occupations (see below). Higher scores indicate less breathlessness in COPD patients.
- Barthel Index (BI) measures performance in feeding, bathing, grooming, dressing, bowel control, bladder control, toileting, chair transfer, ambulation, and stair climbing.
- COPDnet is a care model developed for patients with COPD who have been referred to a secondary care setting after treatment in a primary setting proved to be insufficiently effective. It includes a comprehensive diagnostic and self-management trajectory in the outpatient pulmonary department and identifies relevant treatable traits.
- Canadian Occupational Performance Measure (COPM) helps patients to self-assess performance, prioritize what needs to be addressed, and identify goals.
Strategies are then introduced in order to reduce effort associated with daily routines and to reduce fatigue, shortness of breath, and the work of breathing due to the workload and symptom burden of ADLs. These may involve altering body mechanics or altering the environment. Adaptive equipment recommendations may include items such as a long-handled shoe or sock aide, and durable medical equipment suggestions may include a tub seat for seated showers.
Energy conservation techniques include making larger meals to freeze, letting dishes air dry, grouping task items together to minimize unnecessary searches, sliding rather than carrying items, shopping with someone who can carry grocery bags, or using grocery home delivery services (Koolen et al., 2021; Yamaguchi et al., 2021).
CASE
Stuart Moody is being discharged from the hospital with a new diagnosis of mild to moderate emphysema-type COPD. His physician has ordered that Mr. Moody start a pulmonary rehabilitation program with a follow-up visit in six weeks. The nurse explains to Mr. Moody that a PR program consists of education about exercise training, nutritional counseling, medications, how to self-measure peak flows, breathing training, panic control, and airway control.
Mr. Moody will see a registered dietitian in consultation before discharge to help him maintain or achieve his correct weight. He will also collaborate with a physical therapist to learn progressive aerobic, strengthening, and resistance exercises, and an occupational therapist to learn new body mechanics for easier performance of ADLs.
Mr. Moody is fortunate to be started on PR at this time. Previously, PR was only ordered for moderate to severe COPD. He is also fortunate that Medicare now covers PR.
Pharmacologic Therapies
The medicines currently available for COPD focus on long-term therapy with the goal of preventing symptoms, reducing the frequency and severity of exacerbations, improving exercise tolerance, and improving general health. Stepped therapy—the process by which additional medications are added as symptoms progress—is the standard in treating COPD. Inhaled and systemic drugs for patients with COPD include:
- Beta-adrenergic agents
- Cholinergic antagonists
- Methylxanthines
- Corticosteroids
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Phosphodiesterase inhibitors (in severe cases)
- Mucolytic agents
Drug therapy is used to reduce the extent to which dyspnea restricts a patient’s activities. Most COPD drugs work by keeping airways as wide open as possible. Medications (bronchodilators) used to reduce airflow obstruction are not typically given to asymptomatic patients with COPD.
BRONCHODILATORS
Bronchodilators are the “workhorses” of the COPD medications. Although spirometry shows that bronchodilators only modestly reduce airway obstruction in most patients with COPD, regular doses of bronchodilators relieve dyspnea sufficiently for patients with COPD to increase their levels of activity. Combination treatment increases FEV1 and reduces symptoms and exacerbations more effectively than monotherapy.
Bronchodilators work by relaxing the smooth muscles in the walls of the lungs’ airways. This widens the airways and allows air to move through them more easily. Short- and fast-acting bronchodilators are used as “rescue” medicines to relieve sudden bouts of dyspnea and coughing. Long-acting bronchodilators are used in daily, regularly scheduled drug regimens to prevent airway obstruction. Bronchodilators prevent hyperinflation of the lungs at rest and during exercise and thereby improve exercise intolerance.
All symptomatic patients are prescribed a short-acting rescue bronchodilator that they can use to recover from a bout of suddenly worsening dyspnea. Either short-acting parasympatholytic or short-acting sympathomimetic bronchodilators can be used as fast-relief medications (GOLD, 2021; Harding et al., 2020).
Parasympatholytic Bronchodilators
The parasympatholytic bronchodilators are anticholinergic drugs, which relax the smooth muscles in the airway by blocking the effect of acetylcholine, produced by the parasympathetic nervous system.
The most commonly prescribed short-acting anticholinergic bronchodilator is ipratropium (Atrovent). Ipratropium is relatively inexpensive and widely available. It is usually administered via a metered-dose inhaler (MDI), although there are other formulations. It can be used as a PRN medication. It takes effect in 15–30 minutes, has its peak action in 1–2 hours, and lasts 4–6 hours.
Traditionally, ipratropium has also been used as the main anticholinergic in long-term drug regimens. However, studies have shown that tiotropium (Spiriva) is a more effective drug. Tiotropium is a longer-acting anticholinergic bronchodilator. It is more expensive than ipratropium, but a typical dose lasts an entire day. Tiotropium is helpful when used alone and is even more effective in combination with a long-acting beta agonist. Tiotropium is inhaled as a powder via a dry powder inhaler (DPI) (GOLD, 2021; Harding et al., 2020).
When used correctly, MDIs and DPIs deliver the medication directly to the airway. MDIs work best when connected to a “spacer” to guide the medication down the patient’s airway.
HOW TO USE A METERED-DOSE INHALER WITH A SPACER

Metered‑dose inhaler. (Source: NIAID, 2016.)
Clinicians can provide these instructions to educate patients with COPD on using their MDI:
- Make sure that the metal canister of the MDI is inserted correctly into the plastic “boot” or holder.
- Remove the cap from the mouthpiece of both the MDI and the spacer.
- Insert the MDI mouthpiece into the soft opening of the spacer. The MDI canister must be in an upright position.
- Shake the MDI with attached spacer several times.
- Breathe out, away from the spacer, to the end of your normal breath.
- Place the mouthpiece of the spacer into your mouth, past your teeth, and above your tongue. Close your lips around the mouthpiece. If you are using a spacer with a mask, place the mask over your nose and mouth. Be sure the mask has a good seal against your cheeks and chin; there should be no space between the mask and your skin.
- Press down on the top of the metal canister once to release the medicine into the spacer.
- Breathe in deeply and slowly through your mouth. If the spacer makes a “whistling” sound, you are breathing in too quickly. You should not hear a whistle.
- Hold your breath for 5–10 seconds.
- Breathe out slowly.
- If you are instructed to take more than one puff (spray), wait about 15–30 seconds (or as directed by the package insert) before taking the next puff. Then repeat steps 4 through 10.
- Replace the cap on the mouthpiece of the MDI inhaler and spacer after you have finished.
- If you are inhaling a steroid, rinse your mouth out with water, swish, and spit out the water.
(Harding et al., 2020)
HOW TO USE A DRY POWDER INHALER

Dry powder inhalers (left to right): Turbuhaler, Accuhaler, Ellipta devices. (Source: NIAID, 2016.)
Clinicians can provide these instructions to educate patients with COPD on using their DPI:
- Remove all candy, food, or gum from your mouth.
- Stand up straight.
- Hold the inhaler level to the floor.
- Open the inhaler with the mouthpiece facing you.
- Slide the lever away from you until you hear it click. This means the medicine has been released. Be careful not to tip the inhaler or slide the lever again; if you do, the medicine will fall out and be wasted.
- Take a deep breath in and then breathe out.
- Place the inhaler in your mouth, seal your lips tightly around it, and take a quick, deep breath in.
- Hold your breath for 10 seconds and then breathe out.
(Harding et al., 2020)
Sympathomimetic Bronchodilators
Beta-2 adrenergic agonists are a class of sympathomimetic bronchodilators that act by mimicking the effect of norepinephrine on airway muscles, causing smooth muscles to relax, thereby widening the airways. Muscle tremors, tachycardia, and heart palpitations are the most common side effects of beta-2 agonists, but when the medicines are inhaled (as opposed to taken in oral formulations), the side effects are usually mild.
Short-acting beta-2 agonists are the most commonly prescribed sympathomimetic bronchodilators and include albuterol (Accuneb, ProAir, Proventil, and Ventolin) and metaproterenol (Alupent). These drugs are usually administered via either MDI or DPI. Short-acting beta-2 agonists such as albuterol and metaproterenol take effect in 5–15 minutes and last for 2–4 hours.
Short-acting beta-2 agonists are used as rescue medicines when a patient needs immediate relief from sudden episodes of increased dyspnea. A short-acting beta-2 agonist can also be added to an anticholinergic drug as part of a regular drug regimen.
Long-acting beta-2 agonist bronchodilators include formoterol (Foradil) and salmeterol (Serevent). These drugs are more expensive than albuterol or metaproterenol, but a typical dose lasts for at least 12 hours. Inhalation is the recommended route for administering the long-acting beta-2 agonists (GOLD, 2021; Harding et al., 2020).
Phosphodiesterase inhibitors, or xanthines, are another class of sympathomimetic bronchodilators. They act by stimulating the release of norepinephrine, which then relaxes smooth muscles in the airways of the lung.
Previously, the phosphodiesterase inhibitor theophylline (Elixophyllin, Theo-Dur) was used to dilate airways, stimulate the respiratory centers of the brain, and improve the function of respiratory muscles. Due to its side effects (diarrhea, nausea, poor appetite, weight loss, abdominal pain, headache, sleep disturbances, and cardiac dysrhythmias) and lower efficacy, theophylline is now rarely used.
Two newer phosphodiesterase inhibitors, cilomilast (Ariflo) and roflumilast (Daxas, Daliresp), appear to be safer than theophylline. Roflumilast is used only for severe exacerbations (GOLD, 2021; Harding et al., 2020).
Bronchodilator Regimens
Patients vary in their response to bronchodilators, so the most effective drug regimens are those that have been individually tailored. Finding the right drug or set of drugs is empirical. Patients may try many different inhalers until finding a combination that works best for them. When drug combinations are being tried, it is best to introduce the drugs one at a time to learn the patient’s response to that drug only.
For patients with chronic stable COPD, short-acting bronchodilators will eventually be insufficient to control their symptoms. Currently, the long-acting anticholinergic drug tiotropium is usually recommended as the first drug to try in a regular daily medication regimen. It is taken once daily and it does not have the side effects of sympathomimetic drugs, but it is generally not as effective as the beta-2 agonists and is only recommended if the COPD patient cannot tolerate the beta-2 agonist side effects.
Concurrently, a short-acting beta-2 agonist, such as albuterol, is usually prescribed as a rescue drug. If this initial regimen is insufficient, the short-acting beta-2 agonist is added to the regularly scheduled drug regimen rather than being used only when needed. The combination of ipratropium and albuterol is available commercially (DuoNeb) as an inhalant.
As COPD progresses, most patients do better with combinations of two or three bronchodilators. In American and Western European medicine, theophylline (or another phosphodiesterase inhibitor) is usually the last bronchodilator to be added.
If they are to be followed faithfully, drug regimens must be realistic. Bronchodilator therapy with two or three drugs is expensive. In addition, using inhalers can be physically difficult for some people, especially older adults, and physicians may need to modify an optimal pharmacologic therapy to make it practical for a particular patient (GOLD, 2021; Harding et al., 2020).
BRONCHODILATORS
Short-acting beta agonists
- Action: relaxes bronchiolar smooth muscle
- Drugs: albuterol (Proventil), metaproterenol (Alupent), erbutenol (Maxair), terbutaline (Brethine)
- Side effects: tachycardia, palpitations, anxiety, muscle tremors
- Comments: fast-acting rescue drug; taken 5 minutes before other inhalers; used for treatment
Long-acting beta agonists
- Action: relaxes bronchiolar smooth muscle
- Drugs: salmeterol (Serevent)
- Side effects: tachycardia, palpitations, anxiety, muscle tremors
- Comments: not to be used for acute onset of symptoms; inhaler must be shaken since drug separates; used for prevention
Anticholinergic agents
- Action: inhibits parasympathetic nervous system
- Drugs: ipratropium (Atrovent)
- Side effects: cough, dry mouth
- Comments: may exacerbate cardiac symptoms; inhaler must be shaken since drug separates; used for prevention; must be carried at all times if used as a rescue drug
(GOLD, 2021; Harding et al., 2020)
CORTICOSTEROIDS
Corticosteroids, also called glucocorticoids, are “two-edged swords.” They are effective anti-inflammatory medicines used to reduce the inflammatory response that underlies or exacerbates many diseases. However, the continued use of corticosteroids may cause Cushing’s syndrome, glaucoma, cataracts, myopathy, ulcers, osteoporosis, hyperglycemia, poor wound healing, and the inability to overcome infections.
In stable COPD, the problems that result from the long-term use of oral or systemic corticosteroids usually outweigh the drugs’ benefits. Inhaled steroids—such as fluticasone (Flovent), beclomethasone (Beclovent, Beconase), and budesonide (Pulmicort Turbuhaler)—have fewer adverse effects than oral formulations, and approximately 10% of people with COPD find that regularly inhaled steroids reduce their airway obstruction. For this population of patients, inhaled steroids can be a useful addition to the other regularly scheduled bronchodilators.
The regular use of inhaled corticosteroids is usually reserved for patients with severe COPD. In people with severe COPD, steroids will reduce the number of exacerbations and the rate of mortality. For people with severe COPD, inhaled corticosteroids are typically combined with a long-acting beta-2 agonist in a regular treatment regimen. Regular use of inhaled corticosteroids for COPD, however, also increases a patient’s risk of developing pneumonia.
The usefulness of corticosteroid therapy cannot be predicted in advance for any one patient. Evaluating a patient’s response to the medication by spirometry is the only way to identify in advance those patients with COPD who will be helped by adding inhaled steroids to their regular regimen of bronchodilators (GOLD, 2021; Harding et al., 2020).
ANTI-INFLAMMATORIES
Corticosteroids
- Action: disrupts inflammatory pathways
- Drugs:
- Inhaled: fluticasone (Flovent)
- Oral: prednisone (Deltasone)
- Side effects:
- Inhaled: coughing, hoarseness, dry mouth, sore throat
- Oral: glaucoma, edema, hypertension, mood swings, weight gain, cataracts, hyperglycemia, infections, osteoporosis and fractures, menstrual irregularities, suppressed adrenal gland hormone production, thin skin, easy bruising, slower wound healing
- Comments: must be taken every day, even if there are no symptoms; not to be stopped suddenly; taken with food; reduces local immunity and may increase risk for local infections like candida (yeast)
Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Action: stabilizes mast cell membranes to prevent inflammation
- Drugs: nedocrimil (Tiladel)
- Side effects: dyspepsia, nausea, hyperacidity; in higher doses, MI, CVA, rash, gastrointestinal bleeding
- Comments: must be taken by inhaler every day for prophylaxis, even if there are no symptoms; further testing needed to support the use of nedocrimil in COPD
(GOLD, 2021; Harding et al., 2020)
PREMIXED COMBINATION INHALERS
- Action: combines the effects of bronchodilators and corticosteroids
- Drugs:
- Short-acting: ipratropium and albuterol (DuoNeb), ipratropium and fenoterol (DuoVent)
- Long-acting: formoterol and budesonide (Symbicort), salmeterol and fluticasone (Advair)
- Side effects:
- Short-acting: cough, dry mouth, tachycardia, palpitations, anxiety, muscle tremors
- Long-acting: coughing, hoarseness, dry mouth, sore throat, tachycardia, palpitations, anxiety, muscle tremors
(GOLD, 2021; Harding et al., 2020)
MUCOLYTIC AGENTS
Patients with COPD often have thick, tenacious mucus that is difficult to expectorate, particularly during an acute exacerbation. Mucolytic agents can be given by respiratory nebulizer treatments, sometimes mixed with normal saline to thin secretions. They can also be given orally to produce a systemic effect. Acetylcysteine (Mucomyst) or dornase alfa (Pulmozyme) are commonly given by inhaled nebulizer treatment, and guaifenesin (Robitussin) is given by mouth to promote expectoration.
MUCOLYTIC AGENTS
- Action: thins secretions to promote expectoration
- Drugs:
- Inhaled: acetylcysteine (Mucomyst), dornase alfa (Pulmozyme)
- Oral: guaifenesin (Robitussin)
- Side effects: foul smell, sticky nebulizer mask, white patches or sores inside mouth or on lips, nausea, fever, nasal drainage, sore throat, drowsiness, rash, clammy skin
- Comments: patient must be instructed in home nebulizer use; may interact with some vitamins, minerals, and herbs; may produce moderate improvement in health status and exacerbation reduction
(GOLD, 2021)
COVID-19 AND COPD TREATMENT
Some of the treatments for the COPD patient with a COVID-19 infection are different than those usually employed. Inhaled and systemic steroids are usually avoided for COPD patients with COVID-19 unless airway inflammation is severe because of the increased tendency for pneumonia. Nebulizers are not considered for use because of the dispersal of droplets from contaminated aerosol or the patient coughing. COPD patients have been involved in clinical trials treating COVID-19 patients with new antiviral medications (GOLD, 2021).
ANTIBIOTICS
Studies show that continuous dosing with antibiotics will sometimes reduce exacerbations of COPD for patients particularly prone to exacerbations:
- Azithromycin 250 mg/day per os or 500 mg 3x/week for 1 year (less efficacy for active smokers), or
- Erythromycin 500 mg 2x/week for 1 year
(GOLD, 2021)
VACCINATIONS
People with COPD are at higher risk for serious, even life-threatening complications that are preventable by vaccination. As protection against serious lower respiratory illnesses, people with COPD are advised to receive an influenza vaccination each year. During outbreaks of strains of flu not covered by the annual vaccination, people with COPD should receive prophylactic antiviral treatment such as amantadine (Symmetrel), rimantadine (Flumadine), oseltamivir (Tamiflu), or zanamivir (Relenza). Older adult patients with COPD have decreased risk for ischemic heart disease when they have been vaccinated for the flu for several years.
Pneumococcal vaccinations are also recommended for people with COPD before age 65. A second and even third dose is recommended for people 65 years and older who got their first dose when they were younger than 65 and it has been five or more years since the first dose (CDC, 2018c; GOLD, 2021).
People with certain medical conditions such as COPD are the most likely to benefit from COVID-19 vaccinations. The COVID-19 virus can easily cause severe, life-threatening damage to lungs that are already at risk for respiratory distress, respiratory infection, and severe dyspnea. There has been extensive and conclusive evidence that those who are fully vaccinated against the COVID-19 virus tend to have milder episodes of the disease, and survival rates are much higher (CDC, 2021f).
ANSWERING PATIENT QUESTIONS
Q:Should I get a flu shot if I have COPD?
A:Flu can cause serious problems in people with COPD, and flu shots can reduce your chances of getting the flu. You should get a flu shot every year. In addition, you should have a pneumococcal vaccination, usually every five years, as well as COVID-19 vaccination.
CASE
Rae Ann Evans presents to the urgent care clinic with a fever of 102.5 °F, (tympanic) diaphoresis, severe dyspnea with a respiratory rate of 28, a heart rate of 122, blood pressure of 158/92, and an oxygen saturation of 89% on room air. She is moderately obese. Upon assessment, the nurse auscultates her lungs and finds diminished breath sounds in the bases and expiratory wheezes throughout all fields.
The patient is sitting on the examination table bent forward, audibly wheezing, and using accessory chest muscles to breathe. She displays equilateral expansion of her chest. She states she is coughing up more secretions than usual and that they are dark yellow and thicker than normal.
Ms. Evans is known to have chronic bronchitis-type COPD. She takes acetylcysteine (Mucomyst) to thin secretions to make them easier to bring up and the antibiotic azithromycin (Zithromax) every day to prevent infections, in addition to her daily bronchodilator inhalers. She is diagnosed with community-acquired pneumonia on top of her chronic COPD and given a nebulizer treatment, a prescription for an additional antibiotic to treat her pneumonia, and another prescription for a bronchodilator to be administered by nebulizer.
The clinician demonstrates the nebulizer machine to Ms. Evans and her husband, as they will have one delivered to their home for self-administered treatments until her condition improves. The clinician also discusses the current medication regime and the new additions, then questions the patient for an understanding on taking her meds correctly.
Oxygen Therapy
Supplemental oxygen improves levels of blood oxygenation and reduces the rate at which patients need to breathe. No other medical treatment has proved as effective in improving survival rates of patients with COPD. For people with COPD, supplemental oxygen also slows the rate at which muscles fatigue. These effects make it easier for patients to breathe more deeply and to exercise for longer periods.
Oxygen therapy is expensive and involves special equipment. Therefore, when people with COPD can maintain a blood oxygenation level of PaO2 >55–60 mmHg (an oxygenation saturation of more than about 89%), this is considered adequate oxygenation (unless the patient is symptomatic) and supplemental oxygen therapy is not routinely prescribed.
Long-term oxygen therapy (>15 hours/day) may be needed for stable COPD patients with an oxygen saturation <88% on room air, with or without hypercapnia. Stable patients may also need long-term oxygen therapy with an oxygen saturation <88% with pulmonary hypertension, peripheral edema, or polycythemia (hematocrit >55%) (GOLD, 2021).
A COPD patient’s dyspnea and concurrently poor arterial blood gasses (ABGs), particularly the oxygen saturation, may necessitate the use of noninvasive ventilation. This may be established in the facility or home settings. When there is an acute exacerbation of the respiratory status in COPD, continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) may be used. These devices deliver pressurized oxygen through masks in order to maximize the delivery of needed oxygen deep in the lungs. The timely use of these devices, particularly in the prehospital period, have proved to significantly reduce the need for intubation and ventilation (Abubacker et al., 2021).
High-flow nasal cannula (HFNC) oxygen therapy is increasingly and effectively used in patients with an exacerbation of COPD. The high-flow oxygen works to alleviate significant hypoxia during an exacerbation (Beuvon, 2022).
CONTINUOUS OXYGEN
Eventually, supplemental oxygen will be necessary for patients with COPD. For some patients, oxygen is needed to participate in regular exercise programs. For others, oxygen is needed simply to conduct typical ADLs.
If they live long enough, all patients with COPD lose sufficient lung function to the point that they will be hypoxemic at rest even on an optimal regimen of regular bronchodilator treatments. For these people, continuous oxygen therapy will not prolong their lives and reduce hospitalizations but will relieve or prevent breathlessness while at rest or during moderate activity (GOLD, 2021).
Low-flow (2–3 L/min) oxygen inhaled through nasal cannulas is usually sufficient to raise a COPD patient’s blood PaO2 to 65–80 mmHg (an oxygen saturation of 89%–94%). In addition to increasing survival rates by about 50%, this level of supplemental oxygen lowers the person’s hematocrit toward a normal range, makes sleep easier, and improves exercise tolerance.
Home oxygen therapy is also recommended for patients with COPD with heart failure, pulmonary hypertension, or erythrocytosis (i.e., a hematocrit >56%) even when their PaO2 is >55 mmHg. Some patients who maintain a higher level of arterial oxygen during the day drop to a PaO2 <55 mmHg when they sleep. For people whose hemoglobin desaturates at night, nocturnal oxygen therapy is helpful.
HOME OXYGEN DELIVERY SYSTEMS
Home oxygen can be provided via an oxygen concentrator, compressed oxygen cylinder, or as liquid oxygen. All of these methods can supply an oxygen concentration of 90% or more to the individual and enrich the local environment. Medicare will pay for required durable medical equipment (DME) and gaseous or liquid oxygen for 36 months, with an additional 24 month extension, if necessary. In many cases, Medicare will cover 80% of the cost for supplemental oxygen for patients with desaturation (oxygen saturation <94%) during sleep or physical activity (Medicare.gov, 2021).
Patients usually breathe supplemental oxygen via a continuous-flow nasal cannula. Devices that “conserve” oxygen (reservoir cannulas such as moustache-configured oximizers or oximizer pendants, electromechanical demand pulse delivery devices, transtracheal oxygen delivery) are especially efficient because they provide all the supplemental oxygen early in each inhalation. Some patients who have trouble keeping low blood levels of carbon dioxide can be fitted with facemasks from machines that deliver supplemental oxygen at continuous positive pressure; these systems provide noninvasive positive-pressure ventilation (Harding et al., 2020).
A home system is usually adjusted to deliver oxygen at 2–3 L/min, and in most cases, this will maintain a patient’s oxygen saturation at >89%. For patients who continue to have dyspnea at night, the flow rate is raised by 1 L/min during sleep.
One goal of oxygen therapy is to allow patients to remain active. Inside the home, long tubes can connect the nasal cannulas to stationary oxygen delivery units so patients can move around. For more freedom and to go outdoors, patients can carry portable tanks of compressed oxygen or liquid oxygen. The risks of home oxygen therapy are hypercapnia, oxygen toxicity, and burns secondary to the flammable nature of oxygen (GOLD, 2021).
HAZARDS
Medical. There is a small risk that too high a concentration of inspired oxygen will suppress the respiratory drive (fueled by hypercapnia) of patients with COPD. Long-term low-flow oxygen therapy is probably safest when the amount of oxygen delivered gives the patient a PaO2 of 60–65 mmHg, which is toward the low end of the acceptable range of inspired oxygen.
Physical. Concentrated oxygen is flammable and poses a fire hazard. Patients and their families cannot smoke or use open flames near the oxygen equipment. The long oxygen tubing may also constitute a fall risk.
AIR TRAVEL
Commercial planes maintain an internal air pressure equivalent to 5,000–8,000 feet above sea level. For those patients with COPD whose resting arterial blood oxygen concentration is low (PaO2 <69 mmHg) even at sea level, the cabin concentration of oxygen will usually not be high enough to avoid hypoxemia. The PaO2 should be maintained at >50 mmHg by supplemental oxygen, if necessary, at 3 L/min per nasal cannula or at 31% by Venturi mask. Airlines can provide supplemental oxygen, and some airlines will allow patients to bring small oxygen delivery systems with them, although patients must make arrangements with the airline in advance (GOLD, 2021).
Surgery for COPD
Surgery is risky in people with severe COPD. Postoperatively, many normal patients temporarily have reduced lung volumes, rapid shallow breathing, and an impaired ability to take in oxygen and expel carbon dioxide. These routine postoperative problems add additional stress to the already compromised respiratory systems of patients with COPD. One result is that patients with severe COPD develop postoperative pneumonia 13 times more often than patients with normal lung function. Preoperative antibiotics can reduce the high rate of postoperative pneumonia.
The lack of alternative treatments for severe COPD has led to the development of three surgical procedures that attempt to improve and prolong the lives of patients with COPD. The techniques are lung transplantation, lung volume reduction surgery, and bullectomy.
LUNG TRANSPLANTATION
People with severe COPD are the most common recipients of lung transplants. Candidates for lung transplantation are patients with severe COPD who have exhausted medical therapies and have life expectancies of ≤2 years. The BODE Index is used to estimate a COPD patient’s life expectancy (see box below). Typically, patients should also be younger than 65 years. Three quarters of patients with COPD who receive lung transplants live for ≥2 years after the operation, and many of the survivors have substantially improved abilities to exercise.
BODE INDEX
The updated BODE Index uses four measurements (body-mass index, airway obstruction, dyspnea, and exercise capacity) to assign patients with COPD to 1 of 15 groups, each with a different estimated survival rate.
This mortality prediction index is a multistage scoring system that provides prognostic data in patients with COPD. The BODE index is better than the FEV1 at predicting the risk of death among patients with COPD. The measurements are:
- B: body-mass index
- >21 (0 points)
- ≤21 (1 point)
- O: degree of airflow obstruction (FEV1 calculated by spirometric measurement)
- ≥65% (0 points)
- 50%–64% (1 points)
- 36%–49% (2 points)
- ≤35% (3 points)
- D: amount of dyspnea (using the Modified Medical Research Council dyspnea scale)
- mMRC 0, Dyspneic on strenuous exercise (0 points)
- mMRC 1, Dyspneic on walking a slight hill (0 points)
- mMRC 2, Dyspneic walking level ground; must stop occasionally for breathlessness (1 point)
- mMRC 3, Must stop for breathlessness after walking 100 yards or a few minutes (2 points)
- mMRC 4, Cannot leave house; breathless on dressing/undressing (3 points)
- E: exercise capacity (distance walked in 6 minutes)
- ≥350 meters (0 points)
- 250–349 meters (1 point)
- 150–249 meters (2 points)
- ≤149 meters (3 points)
Four years after a BODE assessment is made, estimated survival rates for long-standing patients with COPD are approximately:
- 18% for a BODE score of 7–10
- 57% for a BODE score of 5–6
- 67% for a BODE score of 3–4
- 80% for a BODE score of 0–2
(Medscape, 2022)
LUNG VOLUME REDUCTION SURGERY
As noted earlier, the lungs of an emphysematous patient become hyperinflated with air spaces that contribute little to gas exchange. The widened chest caused by hyperinflated lungs is difficult for the patient to expand further when attempting to inhale. By removing lung tissue that contains dead air space, surgery can sometimes reduce the patient’s work of breathing by improving airflow obstruction.
In lung volume reduction surgery, the damaged lung tissue is removed from both sides of the chest. As a result, survivors can usually exercise more than they could before the surgery. Those patients who have mainly upper-lung emphysema also have an increased lifespan after this surgery. Exclusion criteria include ≥75 years of age, active smoker, or unable to exercise despite pulmonary rehabilitation (PR).
The major postoperative complication of lung volume reduction surgery is continuing air leakage from the lungs into the chest. Other potential complications are pneumothorax, pneumonia. blood clots, infection, myocardial infarction, dysrhythmias, or the formation of a fistula (COPD.net, 2021).
BULLECTOMY
In some cases, individual large empty air spaces (bullae) can be surgically removed. Typical bullae in a patient with emphysema are a few centimeters in diameter. Occasionally, however, bullae can be huge, taking up as much as a third of the chest space. These giant bullae squeeze the healthier lung tissue and compress the adjacent blood vessels. By removing giant bullae, the remaining lung tissue can re-expand, and some of the circulation will be restored.
As with lung volume reduction surgery, a major postsurgical complication of bullectomy is persistent air leakage. Premedication with antibiotics greatly reduces the postoperative incidence of pneumonia (Harding et al., 2020).