CLINICAL APPEARANCE OF STABLE COPD
The Typical Patient with COPD
The “typical” American patient with moderate to severe COPD is a White female over 65 years of age with a history of smoking at least one pack of cigarettes a day for more than 40 years (NIH, 2017). She complains of general tiredness and becomes short of breath when exercising. Her legs bother her while walking, so she spends most of her time sitting. If asked to exhale quickly, it takes her an unnaturally long time.
Other aspects of the “typical” picture range along a spectrum:
- If this person is on the emphysematous end of the spectrum, she will tend to be thin and have a wide, barrel-shaped chest. She will always feel a great deal of dyspnea. When she coughs, she will not produce much sputum. On chest examination, this person’s breath sounds will be distant and relatively clear.
- If this person is on the chronic bronchitis end of the spectrum, she will tend to be of normal weight or overweight. She will cough frequently and will bring up sputum. On chest examination, her breath sounds will include rales (dry crackles), rhonchi (harsh, wet sounds), and wheezes. A COPD patient with chronic bronchitis has exacerbations usually related to bacterial respiratory infections.
(Harding et al., 2020)
Chief Complaints
Patients with COPD usually present with the complaints of dyspnea and coughing.
DYSPNEA
Dyspnea during mild exercise is the most common reason that people with COPD first seek out a physician. This dyspnea will have appeared gradually over a period of years. The dyspnea of COPD reflects at least two sensations:
- The urge to breathe. Patients with COPD have airway obstruction, and they cannot fully empty their lungs before they need to take another breath. The residual air, which keeps the lungs hyperinflated, dilutes the oxygen content of the newly inhaled air. Thus, these people feel hypoxemic.
- Difficulty breathing. Patients with COPD have hyperinflated lungs. Their chests remain overly expanded in the resting state (i.e., after exhaling). It is difficult for the respiratory muscles to expand their chest farther when attempting to take a new breath. Thus, these people put an unusual effort into breathing.
Sometimes a patient with COPD will come to the healthcare provider reporting that a recent illness has triggered dyspnea. Illnesses, especially respiratory illnesses and infections, worsen dyspnea. If the patient actually has COPD, a careful review of the history of the patient’s exercise tolerance usually turns up evidence of increasing dyspnea before the illness (Harding et al., 2020).
COUGH
While dyspnea is the symptom that most often brings patients with COPD to visit a healthcare provider, coughing is the most common symptom found in patients with early COPD. The cough of COPD is usually worse in the mornings. Early in the disease, the cough produces only a small amount of colorless sputum (i.e., mucus and lung secretions that are expelled into the throat by coughing).
Coughing typically begins earlier in the development of COPD than dyspnea, especially with chronic bronchitis, but unlike dyspnea, coughing may or may not limit the patient’s daily activities; it depends on what the patient needs to do in a day. (For example, if they teach or preach, coughing may interfere with their work.)
Coughing is stimulated by irritation of the bronchial tree. The sudden onset of new coughing is usually caused by irritation from a respiratory infection and is accompanied by fever, tachycardia, and tachypnea. This type of cough typically lasts less than three weeks, although in some people coughs can hang on as long as two months after a respiratory illness. The coughing of COPD, however, occurs intermittently for years (CDC, 2020b).
CASE
Shelley Bradley made an appointment with her family nurse practitioner (FNP) because of increased dyspnea after a viral respiratory infection she came down with in spite of getting her annual flu shot. She told the FNP that she has had a persistent cough for three weeks after the first flu-like symptoms appeared.
Ms. Bradley was diagnosed with COPD four years ago. She quit smoking at that time and has a 32-pack-year history of smoking. She has no signs of infection and undergoes a chest X-ray, which shows no infection and no change in her airway. She is given a prescription for an ipratropium (Atrovent) inhaler to use in addition to her longer-acting salmeterol (Serevent) inhaler. She is instructed to exhale deeply before administering the medication and to hold her breath after each inhalation of the medication. The FNP has her return the demonstration to show she understands proper technique.
Ms. Bradley’s FNP discusses the importance of protecting herself from contracting a respiratory infection in the future. She discusses the availability of pneumonia vaccines and the value of frequent handwashing and avoiding proximity to people with signs of respiratory infections. The FNP discusses Ms. Bradley’s medication regimes before the end of the appointment.
Medical History
HISTORY OF THE CHIEF COMPLAINT
Almost as a rule, the health system first sees patients with COPD when they are in their late 40s to mid-50s and with chief complaints of dyspnea and excessive coughing. In retrospect, their symptoms have been going on for at least a decade, with coughing having shown up first. At one time, the dyspnea had only been noticed during heavy exertion, but eventually it began to interfere with even mild activities. In the late stage of COPD, dyspnea may be continuous even when the person is at rest.
A thorough medical history of a COPD patient includes risk factors, previous medical history, pertinent family history, evidence of genetic factors, history of symptom progression, prior exacerbations and hospitalizations, comorbidities, and support available to the patient (CDC, 2020b).
During the medical history, most patients with COPD state that typical symptoms are exacerbated upon arising, usually in the morning. These symptoms may include, in descending order of occurrence:
- Dyspnea
- Inability to accomplish full expiration, resulting in air trapping
- Sputum
- Cough
- Wheezing
- Chest tightness
The air trapping results in hyperexpansion of the chest (barrel chest).
Many patients with COPD will report that typical respiratory infections are now occurring more frequently, lasting longer, and seeming more severe. Colds bring on shortness of breath, wheezing, and coughing as the most common symptoms (Harding et al., 2020).
SMOKING
The key element in taking the history of a patient with COPD is inquiring about smoking. The first symptoms of COPD appear after about 20 pack-years of smoking, and the disease usually becomes clinically significant after 40 pack-years of smoking (Harding et al., 2020).
OTHER IMPORTANT INFORMATION
Besides chronic diseases and heart conditions, a few other specific problems should be explicitly investigated when taking the history of a patient with COPD:
- Allergy history. Asthma and other allergic syndromes that affect the respiratory system can worsen (or mimic) COPD. There is a great deal of functional and pathological overlap between asthma and COPD, resulting in asthma-COPD overlap syndrome (Harding et al., 2020).
- Symptoms of clinical depression. Depression is more common in people with chronic illnesses such as COPD. Symptoms of anxiety and depression—such as poor appetite, persistent sadness, inability to focus, restlessness, lethargy, poor self-image, somnolence, suicidal ideation, thoughts of harming self, exhaustion, self-loathing, unexplained weight loss, and insomnia—may be found in the medical history and have been treated effectively by pulmonary rehabilitation (CESD-R, 2021; Vikjord, 2020).
Physical Exam
A patient with mild COPD may have no signs of the disease when sitting quietly, and their physical exam may be normal. In contrast, the physical exam of a person with severe COPD can be diagnostic. The physical exam may include measurements of height and body mass as well as spirometry to measure lung function, including forced vital capacity (FVC) and forced expiratory volume (FEV) (Dal Negro et al., 2021). The following may also be included in the physical examination:
GENERAL APPEARANCE
Patients with emphysematous COPD are typically thin but barrel-chested. They tend to breathe through pursed lips, and they sit leaning forward in a “tripod” position, supporting the upper body on the elbows or the extended arms. This posture widens the chest as much as possible by forcing the diaphragm down and forward.
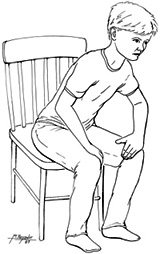
The tripod position. Patient leans forward, resting on elbows or hands, in an effort to expand the chest and ease breathing. (Source: Jason M. Alexander, MFA. © 2007, Wild Iris Medical Education.)
Patients with chronic bronchitis COPD are typically of normal weight or overweight. They have a productive cough and may be cyanotic. At rest, their rate of respirations is high, often more than 20 breaths per minute. Patients may present as dull and irritable because their state of consciousness can be clouded by hypoxemia.
WEIGHT
The patient’s weight will influence the treatment recommendations. Obesity worsens the symptoms of COPD. On the other hand, many patients with COPD, especially patients with the emphysematous form of COPD, are cachectic and underweight, and have wasted muscles. In these cases, nutritional therapy will be important.
CHEST
A patient with COPD with chronic bronchitis but little emphysema may have a normal-sized chest. Significant emphysema, however, leads to a wide, barrel-shaped chest with a flattened diaphragm. In a patient with emphysema, the chest remains perpetually in the position of inhalation. To take a new breath, emphysematous patients must expand their chests beyond the normal position of inhalation. This requires using accessory respiratory muscles of the shoulder, neck, and back.

Normal lateral chest X-ray. (Source: James Heilman, MD.)
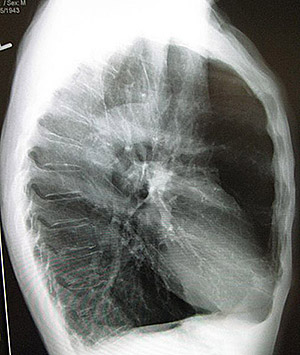
A lateral chest X-ray of a person with emphysema. Note the barrel chest and flat diaphragm. (Source: James Heilman, MD)
LUNGS
The chest of an emphysematous patient is unusually resonant to percussion, and the breath sounds are distant. At the other end of the spectrum, the chest of a chronic bronchitis patient can have dull spots when percussed, and their lungs will be noisy with rales, rhonchi, and wheezing.
The common feature of all forms of COPD is airway obstruction that worsens as the disease becomes more severe. A simple, direct measure of airway obstruction is the time it takes a patient to exhale an entire lungful of air. A normal person has a forced expiratory time (FET) of <3 seconds. An FET of >4 seconds suggests obstruction, and an FET of >6 seconds indicates considerable airway obstruction at the level of moderate-to-severe COPD.
HEART
COPD can injure the heart in two major ways:
- The chronic inflammatory state of COPD predisposes a person to develop coronary artery disease. Therefore, the history and physical examination of a patient with COPD should look for evidence of ischemic heart problems.
- COPD can cause pulmonary hypertension that strains the right ventricle of the heart. Pulmonary hypertension will intensify the pulmonary component of the second heart sound. In addition, pulmonary hypertension can cause tricuspid valve insufficiency, which can be heard as a holosystolic murmur loudest along the left sternal border. When pulmonary hypertension causes right-sided heart failure (cor pulmonale), the patient will have jugular venous distension and edema of the legs and ankles. Cor pulmonale is a late outcome of COPD and causes a poorer prognosis, although not all people with COPD will develop cor pulmonale.
(Harding et al., 2020)
CASE
Lionel Messenger is a 72-year-old man admitted to the intensive care unit following a myocardial infarction. He has a history of type 2 diabetes mellitus, hypertension, coronary artery disease, hypercholesterolemia, cor pulmonale, and COPD. He presently lies comfortably in bed without pain or difficulty breathing on two liters per minute of oxygen by nasal cannula. His cardiac monitor shows sinus tachycardia with a heart rate of 110 beats per minute and occasional premature ventricular contractions (PVCs).
Upon physical examination by the critical care nurse, Mr. Messenger displays clear but diminished breath sounds, a systolic heart murmur, 2+ radial pulses, 1+ pedal pulses, 3+ pitting edema half-way to the knees, jugular vein distension (JVD) while upright, and clubbing of the fingertips. As his condition is stable, he will be transferred to the telemetry unit as soon as a monitored bed is available.
Laboratory Findings
The key chemistry values in a person with COPD are the levels of blood gases (oxygen and carbon dioxide) and the pH of the blood.
BLOOD OXYGEN LEVELS
The severity of a patient’s COPD can be estimated by the degree that the blood gases deviate from normal. In the preliminary stages of the disease, the amount of oxygen in arterial blood is usually within normal limits, measured as its partial pressure (PaO2) (or oxygen tension), with a normal level being 80–100 mmHg.
As COPD worsens, the PaO2 can drop below 60 mmHg. This level signals respiratory distress to the brain, and it strongly activates the respiratory centers. When the PaO2 is below 60 mmHg, a person hyperventilates in an attempt to reverse the hypoxemia by breathing in more air. Unfortunately, hyperventilation due to hypoxemia expels too much carbon dioxide from the bloodstream and causes respiratory alkalosis (a pH imbalance in the blood). Hypoxemia with alkalosis is found in the middle phase of the course of COPD.
In later stages of COPD, the patient does not have the energy to hyperventilate, so carbon dioxide builds up in the blood, with the PaCO2 often reading >50 mmHg. Now the hypoxemia is accompanied by hypercapnia, and the patient develops chronic respiratory acidosis, an ominous sign. Hypoxemia with acidosis is found in the late phase of the course of COPD (OA, 2021).
Arterial Blood Gases (ABGs)
Early in the course of COPD, ABGs do not need to be checked regularly. However, an early set of baseline values should be taken because they can be used as a comparison to evaluate the degree of change brought on by an acute exacerbation.
| ABG | Normal Range |
|---|---|
| (Harding et al., 2020) | |
| pH | 7.35–7.45 |
| PaO2 | 80–100 |
| PaCO2 | 35–45 |
| HCO3 - (bicarbonate) | 22–26 |
| Base excess (BE) | -2 to +2 |
| O2 saturation (sat) | 94%–100% |
Pulse Oximetry
Accurately measuring a person’s blood oxygen tension requires drawing arterial blood and testing it in a laboratory. Pulse oximetry is a quicker, noninvasive way to test blood oxygenation. A pulse oximeter has a small probe that can be clipped onto a patient’s finger or earlobe. Using measurements of transmitted light, the oximeter determines the percentage of the patient’s hemoglobin (Hgb) that is saturated with oxygen.
Pulse oximeters are not as accurate as direct oxygen tension measurements from arterial blood gases, and the percentage of hemoglobin saturation measured by an oximeter is not the same as a person’s PaO2. Nonetheless, the two values are related. A person with a normal PaO2 (80–100 mmHg as determined from blood gases) will have a hemoglobin saturation of 94%–100% (as determined by pulse oximetry). A person with hypoxemia of 60 mmHg will have a hemoglobin saturation of approximately 88%. Normal range of oxygen saturation is 94%–100%, but a person with moderate to severe COPD may run lower-than-normal saturation levels when breathing room air. In COPD, dynamic hyperinflation at the end of expiration leads to lower-than-normal oxygen saturation readings, causing exercise intolerance and exertional dyspnea (Harding et al., 2020).
HEMOGLOBIN AND HEMATOCRIT
Routine blood analyses are not needed to manage most cases of COPD. Some people with severe COPD produce excess red blood cells (polycythemia) in response to their chronic hypoxia. This leads to hematocrit (Hct) readings of >52% in men (normal is 43%–52%) and >48% in women (normal is 37%–48%). Another consideration may be that, since oxygen is transported on the hemoglobin (Hgb) molecule, a low Hgb level will cause a decrease in the amount of oxygen available to the tissue.
ALPHA-1 ANTITRYPSIN (AAT) LEVELS
Patients who develop emphysema at an early age (under 40 years) and nonsmokers of any age who develop emphysema are usually tested for their blood levels of the enzyme AAT. (See also “Alpha-1 Antitrypsin Deficiency” earlier in this course.) Approximately 3% of all people diagnosed with COPD have an undetected AAT deficiency. The deficiency is diagnosed by a blood level of the protein or AAT phenotype, or genetic testing. A serum concentration of A-1 antitrypsin <15%–20% of the normal value suggests the presence of an AAT deficiency (Harding et al., 2020).
Imaging Studies
COPD is a disease that is defined as having structural and functional abnormalities: COPD causes progressively worsened airflow obstruction in the lungs. Therefore, breathing measurements are better diagnostic indicators of the disease than are static pictures of the lung. Nonetheless, imaging studies play a role in evaluating patients with COPD and their pathological processes and physiologic consequences.
The most commonly used images for evaluating and managing COPD are chest X-rays and computed tomography (CT) scans. Other modalities that are sometimes used include magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), electrical impedance tomography (EIT), and optical coherence tomography (OCT).
CHEST X-RAYS
Chest X-rays are used to rule out other causes of airway obstruction, such as mechanical obstruction, tumors, infections, effusions, or interstitial lung diseases. In acute exacerbations of COPD, chest X-rays are used to look for pneumothorax, pneumonia, and atelectasis (collapse of part of a lung).
In its later phases, COPD produces a number of changes that can be seen in chest X-rays:
- When COPD includes significant emphysema, the chest is widened, the diaphragm is flattened, and the lung fields have fainter and fewer vascular markings. Emphysema can make the heart look long, narrow, and vertical, and the airspace behind the heart can be enlarged.
- When COPD includes significant chronic bronchitis, chest X-rays have a “dirty” look. There are more vascular markings and more nonspecific bronchial markings, and the walls of the bronchi look thicker than normal when viewed end-on. Often, the heart appears enlarged.
CHEST COMPUTED TOMOGRAPHY SCANS
CT scans are now the imaging technique of choice for lung evaluations. Helical or spiral CT scans may be used with contrast medium for better visibility. CT scans, especially high-resolution scans, are better than chest X-rays at resolving the details of the lung abnormalities caused by COPD. Specifically, CT scans can help define which areas of a patient’s lungs are predominately emphysematous and which are predominately bronchiolitic. Late in the disease, high-resolution CT scans are used to evaluate patients with COPD who are to be treated with lung volume reduction surgery (Harding et al., 2020).
Lung Function Tests
Pulmonary function tests (PFTs) are used to assess the extent of a patient’s airway obstruction. When COPD is diagnosed, baseline pulmonary function values are recorded. Later tests are used to measure the progression of the disease and to evaluate the effectiveness of treatments. For COPD, the two general classes of breathing tests are measurements of lung volumes and measurements of airflow rates/volumes.
LUNG VOLUME
In COPD, airway obstruction makes it difficult to fully empty the lungs. The air that remains keeps the lungs inflated even after a complete exhalation. This makes it more difficult for a patient to pull in sufficient air during the next breath. As a result, the total air volume contained by the lungs increases, but the effective volume of air (the amount of air actually breathed in and out) decreases.
The effective volume of air is called the vital capacity (VC). VC denotes the largest volume of air that can be exhaled after a full inhalation. Usually, this volume is measured by having a patient take as large a breath as possible and then exhale as quickly and forcefully as possible. With these testing instructions, the result is more accurately called the forced vital capacity (FVC) (Haddad & Sharma, 2021; Harding et al., 2020).
AIRFLOW RATES
Besides limiting the effective volume of air in the lungs, COPD also slows the movement of air inside the lungs. This slowing can be measured directly. Measurements of the rate of air movement during breathing are called spirometric measurements or parameters; more specifically, spirometry measures the volume of air exhaled in a defined period of time (Harding et al., 2020).

A small, handheld spirometry device can be used for quick office or clinic tests. (Source: National Institutes of Health.)
The most common spirometric measurement used for COPD is the 1-second forced expiratory volume (FEV1). This is the maximum amount of air that a patient can breathe out in the first second of a forced exhalation after having taken a full breath.
Spirometry is helpful in evaluating the severity of airflow obstruction in patients with symptomatic COPD. On the other hand, spirometry does not add much to the evaluation of asymptomatic patients with COPD because treatments (other than smoking cessation) are not typically begun until after a patient becomes symptomatic (GOLD, 2021; Harding et al., 2020).
(During the COVID-19 pandemic, the standard method of diagnosing COPD, spirometry, was limited because of the severe amount of coughing and droplet dispersion involved that can spread the COVID-19 virus.)
ANSWERING PATIENT QUESTIONS
Q:What is spirometry?
A:Spirometry measures how much air you breathe and how quickly you can get air into and out of your lungs. Spirometry tests are easy and painless. You breathe forcefully into a tube, and the machine at the other end measures how much air you are moving. Spirometry can detect COPD even before you have many symptoms.
Ranking the Severity of COPD
People with normal lungs can expel most of the air in their lungs within 1–2 seconds. The amount of air forcefully exhaled in the first second (FEV1) is about three quarters of a healthy person’s FVC.
In COPD, airway obstruction restricts the rate of exhaling, and people with COPD cannot get a normal amount of air out of their lungs in one second. People with COPD have FEV1/FVC <0.70. When a person has an FEV1/FVC ratio of <0.70 and a history of more than 20 pack-years of smoking, they can be given a presumptive diagnosis of COPD (Harding et al., 2020).
The four basic stages of COPD are mild, moderate, severe, and very severe. COPD is staged by the degree to which the FEV1/FVC is <0.70 when corrected for the person’s age, gender, and body build.
| Stage | Severity | Spirometry |
|---|---|---|
| (GOLD, 2021) | ||
| GOLD 1 | Mild |
|
| GOLD 2 | Moderate |
|
| GOLD 3 | Severe |
|
| GOLD 4 | Very Severe |
|
| * Predicted FEV1 values adjusted for a person’s age, gender, height, and weight can be calculated from published equations. | ||
Differential Diagnoses, Including Asthma
Dyspnea and chronic cough are the presenting symptoms of a number of conditions other than COPD. These conditions include:
- Asthma
- Pneumothorax
- Pulmonary emboli
- Pneumonia
- Lung infections
- Atelectasis
- Interstitial lung disease
- Sarcoidosis
- Effusions
- Upper-airway or foreign-body obstructions
A patient with COPD may also have other comorbidities such as lung masses, respiratory infections, increased incidence of atrial fibrillation, arterial hypertension, heart failure, and ischemic heart disease.
Most of these conditions can be identified using imaging studies, such as chest X-rays, and clinical signs. Anemia or metabolic acidosis can also cause chronic dyspnea, and both of these can be identified by blood studies. A differential diagnosis depends especially on age of onset, chest X-ray (CXR) and CT scan results, volume and character of sputum, and history of smoking (GOLD, 2021).
ASTHMA VERSUS COPD
Asthma, which is another common obstructive airway disease, is high on the list of differential diagnoses for conditions presenting with both dyspnea and cough. Asthma usually cannot be distinguished from COPD by chest X-rays, clinical signs, or blood studies.
Patients with asthma have hypersensitive airways that are always slightly inflamed, edematous, and filled with immune cells, characteristically eosinophils. Certain inhaled allergens and a variety of stresses can trigger these primed immune cells, causing a flare of the disease (an asthma “attack” or exacerbation) that brings on edema, mucus, and narrowed airways. Like COPD, asthmatic attacks will obstruct airways and impede airflow; but unlike COPD, the airway restrictions of an asthmatic attack can be, at least in young people, quickly and almost entirely reversed by bronchodilators.
As people with asthma age, however, their airway obstruction sometimes becomes more fixed and less reversible. Clinically, these people’s disease begins to share more features with COPD, and the two diseases may be hard to distinguish. People with asthma have 12 times the possibility of developing COPD later in life. Determining which disease is present can be important for a patient’s treatment. For example, the dyspnea of asthmatic patients tends to improve markedly when the patient is given steroids, but the chronic dyspnea of most patients with COPD does not improve following steroids (GOLD, 2021).
Some useful distinctions between asthma and COPD include:
- Asthma usually appears in people <30 years of age, while COPD typically appears in people >40 years of age.
- Asthmatic attacks are reversed quickly and completely by medications, while the symptoms of COPD are reversed only modestly and temporarily by medications.
- Asthma often runs in families, while COPD usually does not.
| Disorder | Symptoms/Relationship to COPD | Smoking a Factor? |
|---|---|---|
| (Adapted from Harding et al., 2020) | ||
| COPD |
|
90%–95% with emphysema with no genetic factor |
| Asthma |
|
Symptoms exacerbated with smoking |
| Lung masses |
|
85% |
| Effusions |
|
Possibly |
| Congestive heart failure |
|
Possibly |
| Pneumonia |
|
Possibly |
| COVID-19 |
|
No, cause is infectious |
COPD AND COVID-19 RISK
Most studies have not shown COPD patients to be at a higher risk for becoming infected with the SARS-CoV-2 virus that causes COVID-19. However, having a clinical diagnosis of COPD significantly increases the odds of poor clinical outcomes in patients who contract COVID-19. COPD patients should thus be considered a high-risk group and targeted for preventative measures and aggressive treatment for COVID-19, including vaccination (Gerayeli et al., 2021).
Patients with COPD are therefore instructed in the importance of protecting themselves from COVID-19 by wearing a mask when indoors or in a crowded situation. However, wearing an N95 mask is not recommended for COPD patients because wearing a tightly fitting N95 may cause additional inspiratory resistance and adversely affect the COPD patient’s respiratory rate, peripheral oxygen saturation, and expired carbon dioxide levels. The standard recommended mask is a triple-layered, surgical-quality mask (GOLD, 2021).