WHAT IS ALZHEIMER’S DISEASE?
Normal aging involves changes throughout the body, and the brain is not exempt. In normal aging, the volume of the brain decreases each year after age 65, with greatest loss in the frontal and temporal lobes and greater loss of white matter than grey matter in cognitively normal older adults. Cerebral blood flow decreases up to 5%–20%, with deterioration of mechanisms that maintain cerebral blood flow with fluctuation in blood pressure.
Age-related neuronal loss is most prominent in the largest neurons in the cerebellum and cerebral cortex. The hypothalamus, pons, and medulla have modest if any neuron or volume losses with normal aging. Age-related neuron loss is likely due to programmed cell death rather than inflammation, ischemia, or other mechanism.
Age also affects neurons with loss of dendritic tree, shrinkage of processes, and decrease of synapses. In some areas, dendritic connections may increase, which may be due to repatterning of the brain invoked to compensation for cellular death. Neurons continue to form new synapses, and new neurons are formed throughout the lifespan, but rates of loss are greater than gains.
Lipofuscin accumulates in certain areas of the brain, particularly the hippocampus and frontal cortex, both of which are associated with memory formation, but the impact of lipofuscin on function is unknown. Neurofibrillary tangles and senile plaques, which are two characteristic lesions of Alzheimer’s disease, occur in certain areas of the brain in normal aging, but to a lesser extent than in Alzheimer’s disease. More than 50% of cognitively normal individuals over age 85 have sufficient plaques/tangle burden to make a pathologic diagnosis of Alzheimer’s disease.
People with Alzheimer’s disease experience impaired connections between neurons and neuron cell death, causing impairments in learning and thinking. Alzheimer’s also causes the surface layer of the cerebrum to shrink, which directly affects a person’s ability to plan, recall facts, and concentrate. This damage most often begins in a subcortical structure known as the hippocampus, the area of the brain associated with formation of memories. It later affects areas in the cerebral cortex responsible for language, reasoning, and social behavior.
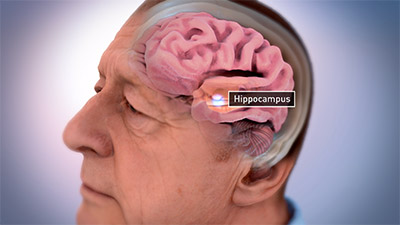
Hippocampus of the brain associated with memory formation. (Source: National Institute on Aging/National Institutes of Health.)
As neurons are injured and die throughout the brain, connections between networks of neurons may break down, and many brain regions begin to shrink. By the final stages of Alzheimer’s, this is widespread, causing significant loss of brain volume (Taffet, 2021).

Shrinkage of the brain due to Alzheimer’s disease. (Source: National Institute on Aging/National Institutes of Health.)
Pathophysiology
The cause of Alzheimer’s disease is poorly understood and includes many environmental and genetic risk factors that are associated with its development. There are several hypotheses for the cause of AD, but the exact cause is still unknown.
Alzheimer’s is largely associated with amyloid plaques, neurofibrillary tangles, and loss of neuronal connections in the brain. Scientists do not know exactly what role plaques and tangles play in Alzheimer’s disease, but it is believed that they disable or block communication among nerve cells and disrupt processes the cells need to survive. The destruction and death of nerve cells causes memory failure, personality changes, problems in carrying out daily activities, and other symptoms of Alzheimer’s.
THE AMYLOID HYPOTHESIS
The amyloid hypothesis was first proposed in 1991 and states that extracellular amyloid beta deposits are the main cause of the disease. In Alzheimer’s disease, plaques develop in the hippocampus, where memories are encoded, and in other areas of the cerebral cortex that are used in thinking and making decisions.
Whether beta amyloid plaques themselves cause AD or whether they are a by-product of the AD process is still unknown (Fan et al., 2020).
THE TAU HYPOTHESIS
The tau hypothesis was introduced in 2009 and proposes that tau protein abnormalities initiate the Alzheimer’s disease process. Tau is mainly found in neuronal axons of the brain, combined with microtubules. The main function of tau is to stabilize microtubules, which is particularly important for neurons since microtubules serve as “highways” for transporting nutrients and neurotransmitters in dendrites and axons.
In this model, a process known as hyperphosphorylation causes tau to pair with other threads of tau into helical filaments, which eventually form neurofibrillary tangles inside nerve cell bodies. When this occurs, the microtubules disintegrate, and the structure of the cells’ cytoskeleton is destroyed. This collapses the neuron’s transport system. Intracellular neurofibrillary tangles (NFTs) are an important pathological feature of Alzheimer’s disease (Fan et al., 2020).
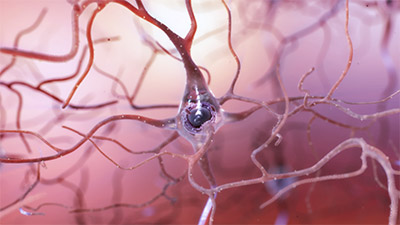
Healthy neuron. (Source: National Institute on Aging/National Institutes of Health.)
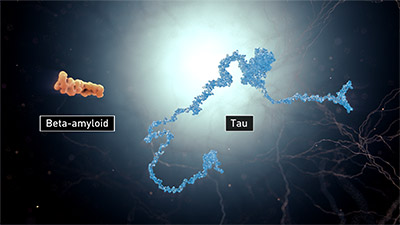
Beta-amyloid and tau. (Source: National Institute on Aging/National Institutes of Health.)
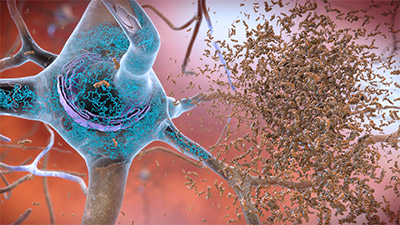
Neuron with beta-amyloid plaques and neurofibrillary tangles. (Source: National Institute on Aging/National Institutes of Health.)
THE CHOLINERGIC HYPOTHESIS
The cholinergic hypothesis is the oldest of all the hypotheses and forms the basis for most of the Alzheimer’s drugs available on the market today. According to this hypothesis, there is a reduced rate of production and transportation of the neurotransmitter acetylcholine in the brains of AD-affected individuals. This neurotransmitter is used by all the cholinergic nerve cells and has an important role in the both peripheral and central nervous systems. Studies have shown the cholinergic system is a crucial contributor to the learning and memory processes (Agarwal et al., 2021).
THE AUTOIMMUNE HYPOTHESIS
The autoimmune hypothesis suggests that Alzheimer’s disease is initiated on a disruption of the blood-brain barrier (BBB) caused by either genetic or nongenetic risk factors. This disruption leads to an autoimmune response against pyramidal neurons located in the structures of the brain involved in memory formation and storage. The response caused by the adaptive immune system is not strong enough to directly kill neurons but may be sufficient to make them selectively vulnerable to neurofibrillary pathology (Arshavsky, 2020).
THE INFECTIOUS HYPOTHESIS
The infectious hypothesis proposes that a pathogen, such as a virus, bacteria, prion, etc., is the root cause of Alzheimer’s disease. This hypothesis is supported by evidence that some pathogens, including herpes viruses and certain bacterial species such as chlamydia pneumoniae and spirochetes, are found more commonly in Alzheimer’s patients. The bacteria are significantly more common in the brains of Alzheimer’s patients who have chronic gum disease (Alzheimer’s Society CA, 2021).
Etiology and Risk Factors of Alzheimer’s Disease
Alzheimer’s is a complex disease with no single, clear-cut etiology and therefore no sure means of prevention or “silver bullet” cure or treatment. Scientists understand that for most people Alzheimer’s is an ecological disease caused by genetics and the interaction of genes with other internal and external factors over many years, leading to changes in brain structure and function. This means that genetics plays an important role, together with how genes are affected by external factors such as environment and lifestyle (epigenetics), some of which are modifiable and some of which are not.
GENETIC RISK FACTORS
The role of genetics in the development of dementia is not yet fully understood, but scientists have found over 20 genes that may increase the risk for the development of Alzheimer’s.
Genes Linked to Early-Onset and Late-Onset Alzheimer’s
There are two types of Alzheimer’s disease, both of which have a genetic component.
Early-onset familial Alzheimer’s disease (FAD). FAD is hereditary and appears well before the age of 65, usually between the early 40s and mid-50s. Studies of families have discovered three known genes that cause early-onset Alzheimer’s, all of which affect processing or production of beta-amyloid:
- Amyloid precursor protein (APP)
- Presenilin-1 (PS1)
- Presenilin-2 (PS2)
There are also cases of early-onset AD that cannot be linked to one of these three genes, suggesting that perhaps there may be other genes not yet discovered (Strobel, 2021).
Late-onset Alzheimer’s. Several genes have been implicated in the risk for late-onset Alzheimer’s, with the APOE-e4 gene exerting the greatest impact on risk. This gene promotes the build-up of beta-amyloid, creating the distinctive plaques seen in the brains of AD patients, and is present in 40%–65% of those with Alzheimer’s. Inheriting the APOE-e2 version of this gene has been found to be a protective factor against AD; however, the mechanism underlying this remains unclear (Alzheimer’s Association, 2021b; NIH, 2021b).
Down Syndrome and Alzheimer’s
In Down syndrome, an individual is born with three copies of chromosome 21 (called trisomy 21) instead of two. People with Down syndrome have an increased risk of developing Alzheimer’s, and this is believed to be related to trisomy 21, which includes the gene that encodes for the production of amyloid precursor protein (APP), which in people with Alzheimer’s is cut into beta-amyloid fragments that accumulate into plaques.
Overall, people with Down syndrome develop Alzheimer’s at an earlier age than people without Down syndrome and by age 40 have significant levels of beta-amyloid plaques and tau tangles in their brains. As with all adults, advancing age increases the likelihood that a person with Down syndrome will develop symptoms of Alzheimer’s (Alzheimer’s Association, 2021b).
TREM2 and Chronic Inflammation in the Brain
It is indisputable that neuroinflammation occurs in the AD-diseased cortex, and animal models and clinical studies strongly suggest that chronic inflammation significantly contributes to Alzheimer’s disease pathogenesis.
In the central nervous system, the TREM2 gene is present on the microglia and is responsible for an exaggerated response of these cells to different irritants (toxic proteins, infectious agents, stroke, depression, hypertension, diabetes, and various neurodegenerative disorders), causing excessive inflammation. Chronic microglial activation leads to excessive neuroinflammation. Genome-wide associated studies have identified variants of the TREM2 gene and linked it with a two- to fourfold increased risk of developing Alzheimer’s disease (Kulkarni et al., 2021).
EPIGENETIC RISK FACTORS
Studies have suggested that late-onset Alzheimer’s disease is driven by epigenetic changes—how and when certain genes are turned on and off—in the brain and how behaviors and environment can cause changes that affect the way genes work. Epigenetic changes are reversible and do not change DNA sequence, but rather change how the body reads a DNA sequence.
Epigenetic regulators have been found to disable protective pathways and enable prodisease pathways in those with AD. Three different epigenetic regulator mechanisms have been identified:
- DNA methylation: DNA methylation regulates gene expression by recruiting proteins involved in gene repression or by inhibiting the binding of transcription factor(s) to DNA.
- Histones: Epigenetic changes alter gene expression without mutation of DNA by influencing production of histones that package and protect DNA. Histones are proteins that bind to DNA, help give chromosomes their shape, and help control the activity of genes.
- Noncoding RNA (ncRNA)–associated gene silencing: A noncoding RNA is a functional molecule that is transcribed from DNA but not translated into proteins. They have been implicated in the deposition of beta-amyloid plaques, the accumulation of neurofibrillary tangles, and the neuroinflammation processes that lead to neuronal death.
(NIH, 2021c; Nativio et al., 2020)
Chronic Stress
There is evidence that chronic stress can accelerate aging, the main risk factor for Alzheimer’s disease. During aging, the functioning of cell glucocorticoid receptors decreases, and free (toxic) cortisol can arise, leading to damaged cerebral areas. It is well documented that stress may affect the memory systems and ability to remember past events. While acute stress is somewhat adaptive and may have beneficial effects on memory functioning in specific situations, chronic stress is associated with a variety of alterations through the production of glucocorticoids, specifically cortisol, that could play a role in decreasing memory encoding and consolidation.
Chronic stress has been reported to accelerate AD pathogenesis, including extracellular beta-amyloid plaque deposition and intracellular tau hyperphosphorylation. The exacerbation of both may be due, at least in part, to excessive secretion of corticosteroids (Avila-Villanueva et al., 2020).
Fructose
New research suggests that Alzheimer’s disease may be driven by the overaction of fructose made in the brain. This helps explain why diabetes and obesity are associated with an increased risk for Alzheimer’s disease. It is proposed that Alzheimer’s is a modern disease driven by changes in dietary lifestyle in which fructose can disrupt cerebral metabolism and neuronal function (Johnson et al., 2020).
Excess Deregulated Brain Iron
Recently, excess deregulated brain iron has been widely reported in the pathogenesis of neurodegenerative diseases, including Alzheimer’s disease. Iron is an essential element involved in many biological processes in the central nervous system, including oxygen transportation, myelin production, and the synthesis and metabolism of neurotransmitters. High concentrations of iron are present in the brains of patients with Alzheimer’s. Excess iron accumulates in the insoluble amyloid plaques and neurofibrillary tangles as characteristics of Alzheimer’s. Elevated neuron iron exacerbates oxidative damage in neuronal cells, ultimately producing the pronounced cognitive deficits in Alzheimer’s disease (Bao et al., 2021).
Diabetes Mellitus
People with diabetes, especially type 2, are at two times higher risk of developing cognitive dysfunction, Alzheimer’s disease, and other dementias when compared to those without diabetes. Much research has been done that indicates a possible connection between diabetes and Alzheimer’s; however, these connections are not completely understood. Multiple factors involved in diabetes-related complications have been found to play a role in the development of neurodegeneration in Alzheimer’s.
Diabetes raises the risk of heart disease and stroke and can cause damage to blood vessels. High blood sugar causes inflammation, which may damage brain cells. Many people with diabetes have been found to have brain changes that are the hallmarks of both Alzheimer’s and vascular dementia, and research has suggested that each condition fuels the damage caused by the other. Many studies suggest dysregulation of insulin levels as a reason behind the development of Alzheimer’s (Mayo Clinic, 2021a; Jash et al., 2021).
Cardiovascular Disease
Studies have indicated that cardiovascular disease and dementia share similar genetic and biochemical profiles and common triggers. Because the brain is highly vascularized, receiving 15% of cardiac output and consuming about 20% of the body’s total oxygen supply, it is particularly vulnerable to impairment of cerebral perfusion. The risk of developing Alzheimer’s appears to be increased by many conditions that damage the heart and blood vessels, including heart disease, diabetes, stroke, hypertension, and high cholesterol.
Hypertension is one of the leading factors. Prior to 2017, high blood pressure was defined as 140 systolic and 90 diastolic. Since 2017, it has been redefined as 130/80. Approximately 46% of the adult population in the United States are now classified as having high blood pressure.
A definite causative mechanism underlying the relationship between hypertension and dementia, and particularly Alzheimer’s disease, has not yet been found. However, it has been suggested that long-standing hypertension, being closely related with endothelial dysfunction, arterial stiffness, and atherosclerosis, is linked with cerebral hypoperfusion (Alzheimer’s Association, 2021b; Tini et al., 2020).
Hearing Loss
Mild, moderate, and severe hearing loss make the odds of dementia 1, 3, and 5 times higher over the following 10 or more years. The associations between hearing loss and the mechanisms underlying cognitive impairment remain unclear. There are several hypotheses in the age-related hearing loss and cognitive decline literature that are based on the premise that hearing loss can alter brain function and structure:
- Common cause: A common pathology could explain the connection, such as that of Alzheimer’s disease or of vascular disease.
- Social isolation model: There is evidence that social isolation increases inflammation and glucocorticoids, which could affect brain structure and has been linked to dementia and cognitive loss.
- Impoverished input: Hearing-impaired individuals experience less cognitive stimulation, which may cause the loss of function in high-level brain areas.
- Increased cognitive load: Hearing loss leads to increased cognitive load on the brain, which in turn increases the risk of cognitive decline. The hearing-impaired individual must use more brain resources than the average person in order to listen, which may reduce the cognitive resources available to do other tasks, and that may lead to cognitive impairment.
Sensorineural hearing loss alone can lead to degeneration of hippocampal neurons. Following hearing loss, decreased neurogenesis is obvious in the subgranular zone (a brain region in the hippocampus where adult neurogenesis occurs), and tau protein phosphorylation is increased in the hippocampus, as is neuroinflammation (Shen et al., 2021; Cruz, 2021).
Depression
Depression is a risk factor for cognitive decline and dementia. Experiencing depression within the past 10 years increases the risk of dementia 4- to 6-fold for older adults. Multiple reports have suggested that a single episode of depression at any point in life is a risk factor for dementia. Chronic depression or recurrent episodes are common in late-life depression, and individuals with persistent depressive symptoms are at an even higher risk for dementia, especially when combined with cognitive deficits.
The explanation for this is hypothesized to be that late-life depression, particularly when it is persistent and treatment resistant, is associated with increased levels of proinflammatory markers, cerebrovascular lesions, and cognitive deficits that may lead to dementia or may speed conversion to dementia by decreasing brain reserve (Brewster et al., 2020).
Head Trauma
Several studies have found that incurring a moderate or severe head injury can increase the risk of developing Alzheimer’s disease many years later. One study found that sustaining a moderate head injury increased the risk of Alzheimer’s disease by 2.3 times, while severe head injury had a 4.5-times greater risk. The mechanism by which this occurs is thought to be linked to cerebral hypoperfusion that leads to the chain of causative events that ultimately result in the hallmark protein-linked brain changes seen in Alzheimer’s (Alzheimer’s Association, 2021b).
Smoking
Smoking is a significant risk factor for Alzheimer’s disease. A large cohort study has shown that the risk of dementia and AD is dose-dependent, increasing with the increasing number of cigarettes smoked. Heavy smoking is associated with a >100% increase in risk of dementia and Alzheimer’s disease after two decades of exposure.
Cigarette smoking is associated with higher beta-amyloid 42 levels (Aβ42), excessive oxidative stress, neuroinflammation, and impaired neuroprotection found in the cerebral spinal fluid. Studies have demonstrated that high Aβ42 CSF levels are strongly associated with Alzheimer’s disease. The primary hypothesis for Alzheimer’s development suggests that Aβ42 promotes plaque formation, accompanied by oxidative stress, cortical inflammation, and neuronal loss, while the abnormal deposition of protein aggregation causes neurotoxicity, cell death, and neurodegeneration. It is understood that the combined effects of oxidative stress and neuroinflammation lead to the accumulation of beta-amyloid (Liu et al., 2020).
Vitamin D Deficiency
Research suggests that people with very low levels of vitamin D are at higher risk for developing Alzheimer’s disease and other forms of dementia. Because the skin’s ability to synthesize vitamin D from the sun decreases with age, vitamin D deficiency is more common among older adults.
A large study has shown that people with extremely low blood levels of vitamin D were more than twice as likely as those with normal vitamin D levels to develop Alzheimer’s or other types of dementia. Other studies have shown no association. At this time, association between vitamin D deficiency and dementia risk is only observational, and more research is needed to show cause and effect (Graff-Radford, 2021a).
Obesity and Inflammation
Obesity, diabetes, and Alzheimer’s disease share several common features, with inflammation emerging as the central link. High calorie intake, elevated free fatty acids, and impaired endocrine function lead to insulin resistance and systemic inflammation. This inflammation triggers neuroinflammation, which eventually interferes with the metabolic and regulatory function of the brain mitochondria, leading to neuronal damage and subsequent Alzheimer’s-related cognitive decline (Khan, 2020).
A recent study done among older adults has found that:
- After an average of 11 years, those who were obese at the start of the study had a 31% higher risk for developing dementia than those with a normal weight.
- Women who carried weight around the waistline had a 39% greater risk of dementia; however, there was no link between waistline and dementia in men.
- Those with both obesity and high waist circumference showed a 28% increased risk of dementia.
- The risk for dementia could not be explained by differences in age, marital status, smoking behavior, or other health conditions, including diabetes and hypertension.
(Ma et al., 2020)
Ophthalmic Comorbidities
A recent study has found that age-related macular degeneration (AMD), cataract, and diabetes-related eye disease (DRED) are independently associated with an increased risk of dementia. The risk for dementia was found to be 26% higher among those with AMD, 11% higher among those with cataract, and 61% higher among those with DRED compared with people without these ophthalmic conditions. Risk for dementia was higher in those with both ophthalmic and system conditions (Shang et al., 2021).
Menopause
Menopause status has been found to be the main factor contributing to higher beta-amyloid levels, lower glucose metabolism, and lower gray and white matter volumes in women. There is growing evidence correlating menopause with risk and progression of neurodegenerative diseases. Loss of estrogen after menopause increases the risk of neurodegenerative diseases, which indicates that estrogen plays an important role in disease onset and progression.
Based on current literature, a decrease in estrogen, progesterone, and insulin-like growth factor-1 (IGF-1) hinders their neuroprotective effects, increases inflammation, and impairs beta-amyloid clearance. Estrogen can regulate beta-amyloid expression, accumulation, and degradation and has been shown to modulate the immune system. Lower estrogen concentration is related to hippocampal dysfunction and poorer performance on initial learning and memory retrieval.
In addition to estrogen, progesterone’s neuroprotective effects are well documented. Circulating progesterone is decreased during menopausal transition and drops dramatically following menopause, reducing its neuroprotective effects. Although these neuroprotective effects are well known, the association between progesterone and neurodegenerative diseases, especially AD, is not very clear.
Estrogen hormones regulate gene expression and maintain their full activity only when IGF-1 levels are in the normal range. If the concentrations of one or both hormones are reduced, estrogens were no longer capable of regulating gene expression. Lower serum levels of IGF-1 are associated with an increased risk of developing AD dementia. Higher levels of IGF-1 may protect against subclinical and clinical neurodegeneration.
Lower serum levels of IGF-1 are associated with an increased risk of developing AD dementia and higher levels of IGF-1 with greater brain volumes even among middle-aged community-dwelling participants. Higher levels of IGF-1 may protect against subclinical and clinical neurodegeneration (Cheng et al., 2021; Anderson, 2020).
GENDER
One’s gender plays a role in the development of late-onset Alzheimer’s disease and involves both genetics and epigenetics. After advanced age, being female is the major risk factor for late-onset Alzheimer’s. Women make up about two thirds of AD dementia patients, and postmenopausal women account for more than 60% of affected individuals.
Factors that may more severely affect women include:
- Family history and APOE genotype
- Depression
- Stroke
- Diabetes mellitus
- Hormone-related risks, including menopause and thyroid disease
- Lifestyle-related factors such as smoking, diet, exercise, and intellectual activity
(Anderson, 2020)
Possible Preventative Strategies
The question of whether Alzheimer’s can be prevented continues to stimulate new research investigations. As of yet, however, there are no clear-cut answers, partially due to the need for more large-scale studies in diverse populations. Although there is no definitive evidence about what can prevent Alzheimer’s disease or age-related cognitive decline, a lifestyle that includes the following elements may be helpful in lowering the risk for development of the disease or slowing its progression.
EXERCISE
Regular physical exercise appears to be one of the best things an individual can do to reduce risk for dementia. Studies addressing the effect of aerobic exercise in middle-aged or older adults have reported improvements in thinking and memory and reduced rates of dementia. Regular exercise can significantly reduce the risk of developing Alzheimer’s dementia by about 45% and other dementias by 30%. Aerobic exercise has been shown to result in a small increase in the size of the hippocampus, which is the equivalent of reversing one to two years of age-related shrinkage.
Exercise in research studies refer to aerobic exercise performed for a sustained period of time, usually 20–30 minutes. Physical exercise, however, can also mean a daily activity such as brisk walking, cleaning, or gardening. One study found that risk of Alzheimer’s disease can be reduced by daily physical tasks such as cooking and cleaning. More research is needed to understand the level and intensity of exercise that is most effective (Alzheimer’s Society, 2021a; Alzheimer’s Association, 2021b).
DIET
Researchers hypothesize that making healthy food choices may improve cholesterol, blood sugar levels, and overall blood vessel health, which may in turn reduce the risk of cognitive impairment or Alzheimer’s disease. Three diets have been studied and found to be beneficial.
- The DASH (Dietary Approaches to Stop Hypertension) diet emphasizes vegetables, fruits, fat-free or low-fat dairy products, whole grains, fish, poultry, beans, seeds, nuts, and vegetable oils. It limits sodium, sweets, sugary beverages, and red meat.
- The Mediterranean diet includes relatively little red meat and emphasizes whole grains, legumes, poultry, fruits, vegetables, fish and shellfish, nuts, olive oil and other healthy fats, and red wine in moderation.
- The Mediterranean DASH Intervention for Neurodegenerative Delay (MIND) diet is a hybrid that combines aspects of both of the above diets. There are five groups of foods to avoid in the MIND diet: sweets, red meat, cheese, fried or fast food, and butter or margarine.
Studies have shown that people who strictly follow any of these three diets had a lower risk of Alzheimer’s disease. In addition, even modest adoption of the MIND diet approach, such as eating two vegetable servings a day, two berry servings a week, and one fish meal a week, appeared to lower the risk of Alzheimer’s disease (Graf-Radford, 2016b).
SLEEP
Neurodegenerative disorders such as Alzheimer’s disease are commonly associated with sleep disturbances. However, a major challenge has been determining the causal relationship between sleep and Alzheimer’s and determining which came first, the sleep disturbance or Alzheimer’s pathology.
During the day, beta-amyloid protein is made in the brain. Decreased sleep increases this production of beta-amyloid and the release of tau, promoting the formation of amyloid plaques and tau pathology. During sleep, the brain cells and their connections shrink, allowing more space between these cells so that beta-amyloid and other substances that accumulate during the day can be flushed away. Research indicates that individuals can reduce their risk of developing dementia by getting six to eight hours of sleep each night (Lucet, 2020; Budson, 2021).
PREVENTING HEAD TRAUMA
Because there is an association between traumatic head injury and an increased risk for Alzheimer’s dementia, it is important to take the following protective steps:
- Wear a seat belt when driving or riding in a motor vehicle.
- Never drive while under the influence of alcohol or drugs.
- Wear a helmet or appropriate headgear when riding a bike or any other open vehicle and when taking part in any sport activities.
- Have a primary care provider or pharmacist review medications for safety concerns, including prescription medicines, over-the-counter medicines, herbal supplements, and vitamins.
- Have vision checked at least once a year.
- Perform appropriate-level strength and balance exercises.
- Make the home as safe as possible to prevent falls.
(CDC, 2021a)
SOCIAL CONNECTIONS
Researchers have found that social isolation among adults ages 50 and older is strongly associated with about a 50% increased risk of dementia. Individuals with large social networks are 26% less likely to develop dementia than those with small networks. Experts are not certain about the reason for this association, but it may be due to direct mechanisms through which social and mental stimulation strengthen connections between nerve cells in the brain.
It has also been noted that living with family members does not decrease one’s likelihood of developing dementia, as this does not provide the right type of social interaction to assure positive cognitive benefits. To be beneficial, individuals must be engaged and participate in social activities outside the family, such as sharing meals, engaging in conversations, playing games, attending lectures, and exercising with others (CDC, 2021b; Charvat, 2019).
FLU AND PNEUMONIA VACCINATION
Flu and pneumonia vaccinations are tied to a lower risk of Alzheimer’s dementia. Having had at least one flu vaccination has been found to be associated with a 17% reduction in Alzheimer’s incidence. Having more frequent flu vaccinations was found to be associated with another 13% reduction.
Vaccination against pneumonia between ages 65–75 reduced Alzheimer’s risk by 25%–30% after adjusting for sex, race, birth cohort, education, smoking, and individual genetic factors. The largest reduction in the risk of Alzheimer’s (up to 40%) was observed among those vaccinated against pneumonia who were noncarriers of the risk gene. More research is needed to explore the biological mechanism for this effect (Alzheimer’s Association, 2020b).
MENTAL ACTIVITIES
Neurologists report that mental exercise can reduce the chances of developing Alzheimer’s disease by up to 70%. Whenever the brain is challenged with new or different tasks, brain function improves. In order for an activity to be considered beneficial, the activity must:
- Engage the person’s attention
- Involve more than one of the senses
- Break a routine activity in an unexpected way (e.g., brushing one’s teeth with the nondominant hand)
Researchers have yet to uncover what elements make brain training interventions effective or which types or combinations of training are required in order to be effective. Mental activities that may have an impact on cognitive decline include:
- Reading and writing every day
- Drawing a map from memory
- Solving mathematical problems in the head
- Learning a new language
- Memorizing a list and testing recall of it
- Playing games involving strategy (e.g., checkers, chess, cards)
- Doing crossword puzzles, Sudoku, or other “brain” games
- Seeking out new activities and unfamiliar settings
- Continuing involvement in educational activities such as attending lectures
- Taking up new hobbies such as learning to play an instrument
- Playing online memory games or video games
- Doing routine activities (such as brushing teeth) with the nondominant hand
- Joining a club or attending social activities
- Volunteering for a cause of interest
(Cherry, 2021; ARPF, 2021)