DIABETES COMPLICATIONS AND FOOT ULCER PREVENTION
The term diabetic foot syndrome (DFS) refers to a cluster of comorbidities that result from diabetes or are made worse by having diabetes. DFS impacts the development of diabetic foot ulcers, infections, and amputations. This syndrome includes Charcot osteoarthropathy, peripheral neuropathy, and peripheral arterial disease as well as limited joint mobility and poor nutrition (Baranoski & Ayello, 2020).
Peripheral neuropathy and Charcot osteoarthropathy (Charcot foot) are two conditions that play a critical role in the development of diabetic foot ulcers, and clinicians must be watchful for the presence of these conditions when working with patients who have diabetes. The early symptoms of Charcot foot are frequently subtle and can easily be missed, with devastating consequences. Therefore, early diagnosis and treatment of these complications is critical in the prevention of DFUs.
Peripheral Neuropathy
Peripheral neuropathy is one of the most frequent complications of diabetes and reported to occur in about 50% of patients with diabetes. It is also the most common predisposing factor to the development of diabetic foot ulcers. Diabetic peripheral neuropathy (DPN) is a serious condition that can greatly diminish the quality of life for those who have it (Abbas & Bal, 2019; WOCN, 2021).
Peripheral neuropathy is a condition in which there is damage to the peripheral nervous system, the complex communication network between the central nervous system and the other areas of the body, including the feet. This damage inhibits the regular neurologic activity of the lower extremities. The exact cause of peripheral neuropathy has not yet been clearly defined, but ongoing studies show that it is related to abnormal metabolic actions that can lead to cell injury and nerve ischemia (Baranoski & Ayello, 2020).
Data indicate that DPN is frequently not sufficiently treated, and the role of enhanced glycemic control as part of the treatment protocol, especially in type 2 diabetes, is not yet fully understood (Wang et al., 2021). Some studies demonstrate a relationship between painful DPN and severe vitamin D deficiency. Vitamin D is believed to preserve the balance between inflammation and immune suppression. Research indicates that increased inflammation in chronic conditions such as DPN is linked to insufficient vitamin D (Xiaohua et al., 2021; Dupuis et al., 2021).
DPN normally progresses slowly. It often remains asymptomatic and is detected only with detailed examination. The majority of those affected can be symptom-free, and most patients with diabetes who have DPN are not aware of the condition. It can also be the first presenting symptom of previously undiagnosed diabetes, with tingling and a feeling of numbness being the two major presenting symptoms (WOCN, 2021).
EVALUATING FOOT SENSATION AND REFLEXES
One of the first assessments a clinician performs for every patient with diabetes is to evaluate sensation in the feet. Even if the patient does not present with any of the signs and symptoms of neuropathy, loss of protective sensation may still exist. The clinician explains to the patient that simple, noninvasive testing for foot sensation requires no special preparation on the part of the patient and can be performed in the primary care provider’s office or clinic as well as at home.
The American Diabetes Association recommends that all individuals with diabetes have a yearly foot exam that includes screening for diabetic neuropathy. Neuropathy screening should include a 10-monofilament test and at least one additional screening procedure (ADA, 2018a).
The Semmes-Weinstein monofilament test is one of the easiest methods for evaluating loss of sensation in patients with diabetic peripheral neuropathy. The testing instrument is made up of a set of monofilaments ranging in thickness and diameter. It is recommended to obtain the monofilaments from suppliers who sell calibrated instruments, since research has found significant variability among different brands of monofilaments. Some brands of monofilaments will bend at 8 g of force instead of at the 10 g of force for which they intended (Baranoski & Ayello, 2020).
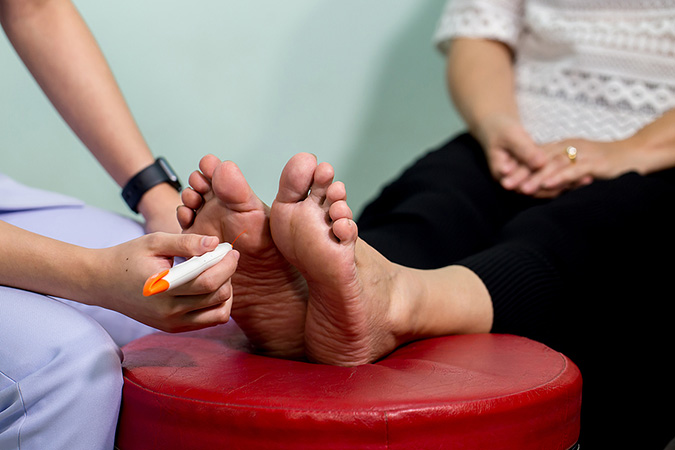
Clinician examining nerve response with a monofilament instrument. (Source: www.bigstock.com, © kckate16.)
SEMMES-WEINSTEIN MONOFILAMENT TESTING PROCEDURE
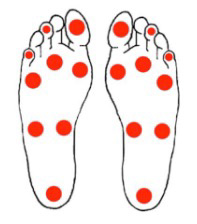
Monofilament testing sites on the bottom of the foot. (Source: U.S. DHHS.)
- Choose a quiet and relaxed setting to carry out the testing.
- Help the patient to assume a comfortable supine position, with shoes and socks removed.
- Explain the procedure to the patient and address any questions.
- Show the patient the monofilament and touch their arm or the back of their hand with it, demonstrating a “C” shape. (The Semmes-Weinstein 5.07 monofilament exerts 10 g of force when bowed into this “C” shape and placed against the patient’s skin for one second.) This demonstration reassures the patient that the test will not be painful and also allows them to experience the normal sensation they should feel when the monofilament touches different points on their toes and feet.
- Instruct the patient to say yes each time they feel the monofilament touch their toes or feet. (Do not ask the patient, “Do you feel that?,” since this question may lead the patient to believe that the expected response is yes.)
- Instruct the patient to close their eyes during testing, explaining that this ensures they are feeling the filament rather than seeing it touch their feet. Also instruct the patient to keep their toes pointed straight up during the test.
- Hold the monofilament perpendicular to the patient’s skin and, using a smooth motion, press down until the monofilament bends into a “C” shape, holding that position for 1–2 seconds before lifting off the filament from the skin surface. The patient should sense the monofilament by the time it bends. If the patient responds yes (that they feel the monofilament), ask the patient whether they feel the pressure on the right or left foot.
- Do not slide the monofilament across the patient’s foot or touch either of the patient’s feet with one’s hands during the testing, so that the only touch that the patient is exposed to is that of the monofilament.
- Apply the monofilament three times to each test site. Also “simulate” one test without applying the monofilament. Avoid repetitive testing at the same site.
- Do not test over areas of callous formation since it is impossible for the patient to accurately determine feeling in those areas.
- For a patient who may already have a diabetic foot ulcer present, test along the margins of the wound and not directly over it.
(Baranoski & Ayello, 2020; WOCN, 2022)
The Ipswich Touch Test (also referred to as the touch the toes test) is another simple, fast, and easy-to-teach method to detect loss of protective sensation in the diabetic foot. The value of this test is that the clinician can instruct the patient or family members to use it at home in order to monitor the presence or absence of sensation. Studies show that when compared to monofilament testing, the Ipswich Touch Test has good predictive value in detecting loss of protective sensation.
The test is done by lightly and briefly touching the tips of the first and fifth toes of the right foot, followed by the same light, brief touch to the first and fifth toes of the left foot, and finally the third toes of the right and left feet. If the patient does not detect the touch at two or more toes, protective sensation has been lost to a large degree. However, one Ipswich Touch Test is usually not enough to determine the percentage loss to protective sensation (Kanza Kazmi et al., 2021).
Other methods to detect loss of protective sensation include reflex testing, pinprick testing, and vibration perception threshold (VPT) testing.
Reflex testing concentrates on evaluating ankle reflexes, since they are regarded as the most sensitive of the lower extremity reflexes when it comes to screening for neuropathy. To perform reflex testing, the clinician ensures that the ankle is in the neutral position and then strikes the Achilles tendon with the examination hammer. An abnormal finding is present if the ankle plantar flexion response cannot be elicited. Abnormal ankle reflexes, combined with an abnormal vibratory or sensory response, is considered a decisive sign of peripheral neuropathy (WOCN, 2022).
Vibratory sense testing can also determine a patient’s sensory perception, with a patient’s inability to sense vibration from a standard tuning fork being indicative of sensory neuropathy. Studies propose that such loss of vibratory sense often happens prior to a patient’s loss of protective sensation and that testing for vibratory sense at the onset of care allows the clinician to discover neuropathy at an earlier stage, when interventions may prove more effective.
The mechanism for vibratory sense testing involves a straightforward procedure using a 128-Hz tuning fork. The clinician strikes the tuning fork on an object or their own hand and then places the tip of the vibrating fork at the patient’s first metatarsal bone at the base of each great toe, instructing the patient to indicate when they can no longer feel the vibration. Test results are regarded as abnormal if the patient can no longer feel the vibrations but the clinician can (WOCN, 2022).
Vibration Perception Threshold testing uses a hand-held device that provides quantifiable results and is comparable to testing with a tuning fork. After explaining the procedure to the patient, the clinician places the oscillator flat against the patient’s foot, applying only enough force so that the surface of the oscillator makes contact with the skin. The amount of vibration is gradually increased until the patient is aware of it, and at this point the reading on the meter is noted. Testing is not done over areas of calluses or scars but can be done along the margins of existing wounds and necrotic tissue if present (Baranoski & Ayello, 2020).
TYPES OF NEUROPATHY
Neuropathy has been broadly classified into three types—sensory, motor, and autonomic—with corresponding characteristics and interventions.
Sensory Neuropathy
Sensory neuropathy leads to the loss of protective sensation, the warning mechanism that lets individuals know that corrective action is needed. When normal sensation is present, feet are immediately removed from danger, but with sensory neuropathy, the awareness of danger is no longer present. For example, a patient may not notice having stepped on a sharp object that has become lodged in the foot or having sustained severe burns by submerging the feet in scalding hot water.
Sensory nerves also conduct messages from the central nervous system to the extremities relating to position. Thus, clinicians may find that patients with DPN have difficulty managing certain movements such as keeping their balance or walking with their eyes closed, which creates a great fall risk for these patients (WOCN, 2022).
Sensory neuropathy frequently begins with numbness and paresthesia (an abnormal sensation of burning or tingling) in the toes and feet and progresses up the patient’s leg in what is referred to as a “stocking” pattern of sensory loss. Eventually, the fingers and hands become affected also, with a loss of sensation moving up the arms in a “glove” pattern.
When the clinician examines the patient’s feet, they also assess for loss of feeling or sensations of “pins and needles” in the patient’s fingers and hands in order to better determine how far the sensory neuropathy has advanced.
Prior to a loss of protective sensation, the patient often experiences several years (in many instances up to 10 years) of painful neuropathy. In its mildest form the patient will refer to these sensations as “pins and needles” or “tingling.” As DPN progresses, the patient often describes “burning,” “stabbing pain,” or “electric shock” feelings. The patient may also complain of itching, heightened sensitivity to generally painless stimuli such as bed sheets on their feet, or an excessive reaction to painful stimuli (WOCN, 2022).
Relief from neuropathy pain comes from movement, and so pain is usually worse at night when the patient is lying down. Patients will complain of interrupted sleep related to neuropathy pain, cramping, restless legs, and the need to move their legs to get relief. The combination of pain and a lack of sleep frequently precipitates exhaustion and depression.
When determining the origin of lower extremity pain in a patient with diabetes, one of the first questions the clinician asks is what relieves the pain? Ischemic leg pain due to poor arterial circulation is relieved by rest and lowering the extremities (dependent position), whereas neuropathy pain is relieved by the patient getting up and walking around.
Motor Neuropathy
Motor neuropathy is found in the muscles responsible for normal movement of the feet. Distal motor nerves in the foot are most frequently involved, resulting in muscle atrophy. Changes in muscle bulk alter the shape of the patient’s foot, causing deformities that affect the patient’s ability to walk and to bear weight. Over a period of time patients with motor neuropathy can lose up to half of the muscle volume of their feet (WOCN, 2022).
The visible signs of motor neuropathy are an abnormal gait pattern and irregular weight-bearing that causes areas of excessively high pressure on the soles of the feet. Other signs of motor neuropathy are rigidity in the patient’s ankles and toes, which is also related to atrophy of the intrinsic muscles.
The structural deformities that result from motor neuropathy have a major impact on the patient with diabetes, including balance, ambulation, and the heightened risk for ulceration. Some of the main structural deformities that occur are:
- Hammertoe (“claw toe”): A flexion deformity resulting in a contracture of the proximal joint of one or more of the smaller toes
- Mallet toe: Similar to a hammertoe, but the flexion contracture occurs at a distal joint in the toe
When examining the foot of a patient with motor neuropathy, the clinician often finds unusually prominent metatarsal heads and claw toes. These deformities are due to shortening of the Achilles tendon. The disproportionate prominence of the metatarsal heads causes two problems for the patient: abnormal weight-bearing and a high possibility of shear and pressure injury to the metatarsal heads. This can lead to callous formation and the development of foot ulcers. Foot drop is another complication of motor neuropathy (Zubair et al., 2021).
Autonomic Neuropathy
Autonomic neuropathy is a condition of the autonomic (involuntary) nervous system, which is responsible for the regulation of many body functions such as temperature control, sweating, and the width and tone of blood vessels. Problems with the autonomic nervous system can affect many parts of the body. It is a condition that is usually associated with long-standing diabetes.
A patient with diabetes who develops autonomic loss of control of the sweat glands will present with extremely dry feet, progressing to the development of fissures (either partial-thickness or full-thickness narrow cracks or openings in the skin). These openings are difficult to treat and keep clean and provide a portal of entry for bacteria, which can result in serious infection. Usually, the loss of sweating spreads from the feet up to the patient’s knees, and the clinician will notice dry, flaking skin on the lower legs (Zubair et al., 2021).
The deregulation of blood vessels common in autonomic neuropathy leads to continuing dilation of the arteries in the feet because there is a lack of sympathetic innervation, which allows for partial constriction of blood vessels when the feet are at rest. During examination, the clinician looks for distended veins over the dorsum of the foot and in the ankle areas. These are a result of arteriovenous shunting, which is a definitive sign of autonomic neuropathy in the feet (WOCN, 2021).
ANSWERING PATIENT QUESTIONS
Q:If I develop a problem with my feet related to my diabetes, won’t I notice some pain?
A:Not necessarily. If a person has diabetic sensory neuropathy, they may have lost a great deal, or even all, feeling in one or both feet. People with diabetes are not always aware of this, and injury can happen due to poorly fitting shoes or a foreign object in the shoe without the person noticing any pain or discomfort.
TREATING DIABETIC NEUROPATHY
Patients with neuropathy are referred to a neurologist for a full evaluation. Currently, there is no treatment that will prevent or reverse DPN. Interventions to treat DPN include medications, devices, exercise, medical foods, genetic/biological therapies, revascularization, hyperbaric oxygen therapy, acupuncture, massage, and lifestyle modifications.
MEDICAL FOODS
The term medical food is defined as “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for a specific dietary management of a disease or condition for which distinctive nutritional requirement, based on recognized scientific principles, are established by medical evaluation” (U.S. FDA, 2017).
Drug treatment is by far the most common intervention and includes antidepressants, anticonvulsants, topical preparations, opioids, and nonopioid pain medications. Within drug therapy, the most widely used drugs in the treatment of DPN are the anticonvulsant medications pregabalin, gabapentin, and carbamazepine (Wang et al., 2021).
Some studies have found that treatment with pregabalin and duloxetine have resulted in an improved quality of life for patients with painful diabetic neuropathy. Pregabalin is the drug that has received the most attention, and studies show that it has a positive impact on neuropathic pain in approximately 30%–50% of patients, with the therapeutic range for the drug between 150–600 mg/day. In patients 65 years and older, doses are started lower and titrated more slowly (Pop-Busui et al., 2017).
Although opioids have proven effective in treating neuropathic pain, they are not a drug of first choice due to the high risk of addiction, abuse, and sedation. Opioids are recommended only as an “add-on” therapy when all other drug combinations have failed and the patient has been referred to a specialized pain clinic for more intensive monitoring (Pop-Busui et al., 2017).
In its position statement on diabetic neuropathy, the ADA emphasizes the importance of screening and early recognition and intervention. The most important interventions in combating all types of neuropathy are tight glycemic control, patient education, and close monitoring for progression of symptoms. In the presence of DPN, daily foot care and inspections become more important. The clinician explains to the patient that once protective sensation is lost, their eyes now become the barrier to injury (Pop-Busui et al., 2017).
Treatment of diabetic neuropathies also requires the skills of all members of the diabetic foot care team. Neuropathy can lead to a progressive loss of proprioception, weakness, and impaired balance. Unsteady gait and pain can make it increasingly difficult for patients to perform activities of daily living (ADLs). Physical therapists are instrumental in training patients in safe ambulation (possibly with an assistive device), and occupational therapists will provide education in the use of any equipment recommended to assist with ADLs. Studies have found that for patients who have limited capacity for exercise, low-intensity aerobic therapy can lead to better sensation in the feet and decrease pain and tingling sensations in the lower extremities (Johnson & Takemoto, 2019).
Exercise has been shown to decrease pain related to motor neuropathy and includes a comprehensive program of strength training, exercises to improve balance, flexibility exercises, and aerobic exercises. The physical therapist individualizes the program to match patient needs and may also recommend splints or braces, if appropriate, to improve patient balance and posture (Quan, 2020).
Charcot Osteoarthropathy
Charcot osteoarthropathy is one of the most serious complications of diabetes. Early recognition and prompt and aggressive treatment are necessary to prevent progression of the condition and possible limb amputation.
Charcot osteoarthropathy, frequently referred to as Charcot foot, has been described in nonmedical terms as a condition in which the bone crumbles. Charcot causes bone destruction in the affected foot that has been compared to what ensues in osteoporosis. Dr. Jean-Martin Charcot, a neurologist, first diagnosed the condition in 1868 in patients with tertiary syphilis. However, in 1936 the condition was also recognized in patients with diabetes (Zubair et al., 2021).
Research shows that almost 13% of persons with diabetes who have a loss of peripheral sensation and automatic neuropathy are likely to develop Charcot foot disease (WOCN, 2022). Diabetic foot ulcers also develop in almost 50% of those diagnosed with Charcot foot disease (Boulton et al., 2018). Fortunately, greater awareness of the signs and symptoms of Charcot foot has resulted in earlier intervention.
Charcot foot many times begins with a slight injury to the joint that may not be noticed by the patient, such as stepping off the curb the “wrong” way, resulting in a minor ankle sprain.
Because the presenting symptoms are similar, Charcot foot is often misdiagnosed as cellulitis and treated with antibiotics, which does not address the underlying condition. This may happen with clinicians who are not familiar with Charcot foot when they observe an extremity that has the classic signs of infection (swelling, redness, pain, and tenderness). However, if the patient has diabetes, these signs should arouse a high suspicion of Charcot foot.
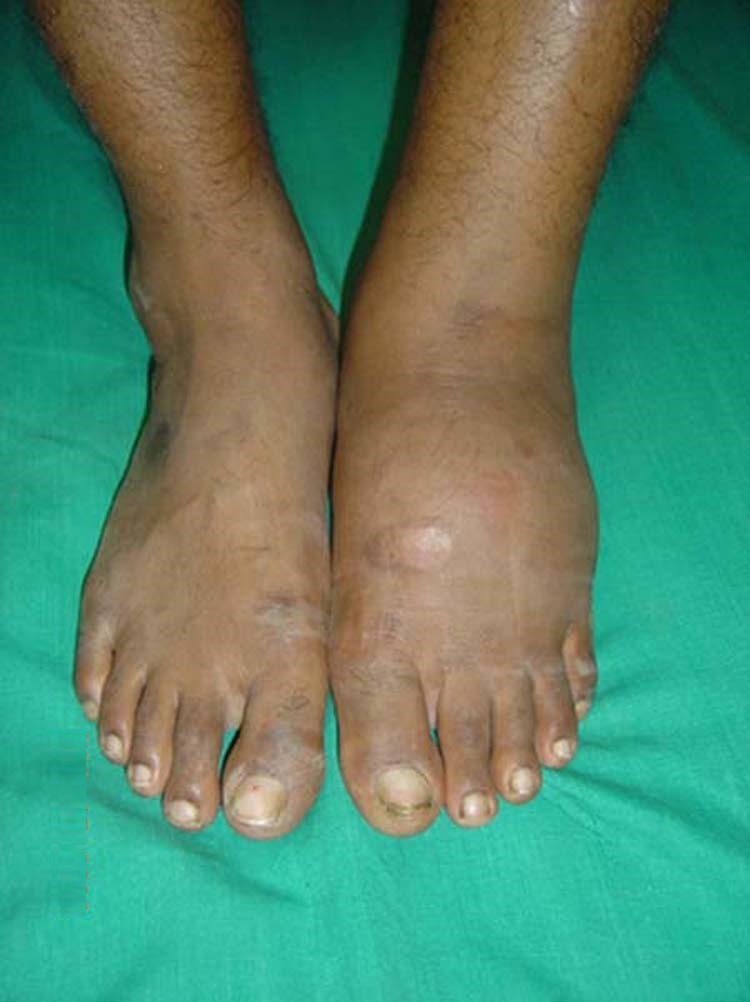
Typical Charcot foot presentation, with diffuse and nonpainful swelling in the left foot. (Source: J. Terrence Jose Jerome. Creative Commons Attribution 3.0.)
STAGES OF CHARCOT OSTEOARTHROPATHY
Charcot osteoarthropathy has been divided into three stages:
- Acute or developmental
- Coalescence
- Reconstruction
(AOFAS, 2021)
In the acute phase of Charcot foot, the patient presents with a foot that is red, swollen, and warm to the touch. There can be a difference in temperature between the affected limb and the nonaffected limb of >2 °C (3.6 °F). It should be noted that pain with Charcot foot is usually less than that experienced with cellulitis (Zubair et al., 2021).
Foot X-rays may show bone crumbling, but there is also a possibility that X-rays will be normal at this early stage of the condition. A bone scan can be useful in discovering focal areas of bone damage. An important sign that the clinician looks for while examining the patient’s foot is a decrease or resolution of redness when the foot is elevated (Zubair et al., 2021). Other signs that the clinician observes in the early presentation of Charcot disease are:
- Swelling of the foot and ankle on the affected side
- Palpable, bounding pedal pulses
- Diabetic peripheral neuropathy with loss of protective sensation
Laboratory studies typically used to diagnose the presence of infection or an inflammatory process (such as a white blood cell count, C-reactive protein, and serum uric acid) are all normal in the acute stage of Charcot foot. Pulses in the extremity affected with Charcot osteoarthropathy are frequently strong and bounding due to the shunting of the circulation in the foot.
There may be several weeks or months between the onset of symptoms and diagnosis. However, if diagnosis is missed at this stage, it can result in further bone destruction and irreversible damage to the effected extremity (AOFAS, 2021; WOCN, 2022).
As Charcot foot progresses, pathophysiological changes are more readily seen on X-ray. These changes include deformity, dislocation, partial dislocation of joints, and bone fractures.
The coalescence stage is noticeable for repair of the extremity, which involves a decreased swelling of soft tissue, the production of bone callus, and the healing of fractures.
Remodeling of bones takes place in the reconstruction phase. This final phase is signified by the occurrence of ankylosis of joints and hypertrophy of involved bones. Clinical findings include the development of a foot deformity, unsteadiness, and abnormal function of the affected joint. The arch of the foot collapses, with the development of what looks like a “rocker bottom” (AOFAS, 2021; Zubair et al., 2021).
Charcot foot can lead to severe deformities that not only alter the patient’s gait but also create areas of high pressure on the surface of the foot, prompting the development of a diabetic foot ulcer. During examination of the patient’s foot, the clinician looks for significant deformity signifying collapse of the structures of the mid-foot and the development of rocker-bottom foot (WOCN, 2022).
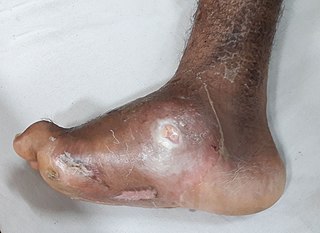
Charcot foot deformity with an ulcerated abscess. (Source: Wikimedia Commons, Creative Commons Attribution-Share Alike 4.0.)
TREATMENT OF CHARCOT FOOT
The appropriate treatment for the patient depends on how far the condition has progressed, the degree of foot deformity present, and which joints are involved. Off-loading and redistributing pressure over the surface of the foot is the cornerstone in the treatment of Charcot foot, preventing the occurrence of diabetic foot ulcers, and healing an existing ulcer (AOFAS, 2021).
The goal of care at this stage is to decrease inflammation and edema and to limit further bone and joint destruction. Non-weight-bearing is the best way to achieve this. This involves immobilization of the extremity. Total contact casting is considered the gold standard for off-loading Charcot foot (Zubair et al., 2021).
Total Contact Casting (TCC) for Charcot Foot
The total contact cast is applied according to the manufacturer’s instructions by a clinician who has been trained in the technique, since an improperly applied or removed cast can cause unforeseen pressure and lead to ulcer formation. TCC is applied to the whole surface of the foot and usually extends to just below the patient’s knee (Zubair et al., 2021). (See also “Total Contact Casting” later in this course under “Off-loading.”)
Typically, the first cast is removed one week after placement to allow inspection of the foot. Thereafter, the cast is usually changed every two weeks. Casting and limited weight-bearing will continue until the inflammation has resolved.
During each cast removal the clinician carefully examines the affected foot for signs that indicate a decrease in inflammation, including decreased swelling, redness, and temperature. If a skin thermometer is available, a skin temperature in the affected foot that is within 2 °F of the contralateral limb is regarded as being significant for resolution of inflammation. X-rays of the affected foot should be done on a monthly basis until bone callus consolidation is evident. It can take 12–16 weeks to reach this stage of recovery (Bryant & Nix, 2016).
Prefabricated Pneumatic Walking Brace
Not all patients are candidates for total contact casting. The most important consideration is adequate circulation to the extremity. An alternative to TCC is a prefabricated pneumatic walking brace (PPWB) fitted with a custom insole (WOCN, 2022).
PPWBs have the advantage that they are easy to remove, have a high satisfaction rating with patients, and have not been associated with any major complications. They are made from a lightweight, partially rigid casing with an inner lining that supports the patient’s lower leg on the affected side to a few inches below the knee. They are usually secured in place with buckles and Velcro straps. However, they have limited use in patients with severe foot deformity. A further drawback is patient compliance, since this is a device that can be removed at home (WOCN, 2022).
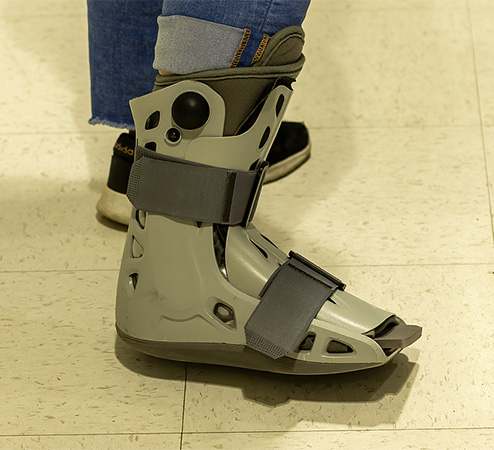
Pneumatic walking brace/boot. (Source: www.bigstock.com, © Karel Bock.)
Postacute Interventions
When the stage of acute inflammation has resolved, total contact casting can be discontinued and the patient fitted for a special brace or footwear to protect the foot. A referral to an orthotist and possibly a pedorthist will be necessary. The goal at this stage is to provide continued stabilization to the foot and ankle while the patient returns to full ambulation. Options for limb protection include a patellar tendon–bearing brace, specialized shoes with modified ankle-foot orthosis, or a Charcot restraint orthotic walker (also known as a CROW boot).
The CROW boot was created for patients who have significant foot and ankle deformity due to Charcot disease. It is fully lined, has a custom foot insert, and includes a rocker bottom sole. The CROW boot can be modified to address changes in the patient’s foot; this is usually achieved by adding or trimming padding where and when appropriate (PFA, 2021).
Assistance from various members of the diabetic foot care team is critical at this stage, especially from the physical therapist and the occupational therapist. The patient will require training on ambulation with the new device, how to correctly put it on and take it off, and how to care for it. Although the specialist who fitted the device will have given the patient instructions, it will be up to other clinicians to reinforce the teaching and to assess for any problems the patient may be encountering with the device.
Ongoing care includes lifetime monitoring of the affected limb, optimum glycemic control, and patient education on the importance of preventing further injury. Once Charcot deformity is present, patients must be made aware of the increased risk for the development of a diabetic foot ulcer. As well as performing the regular components of their routine foot checks, the patient is also taught to check for “hot spots” along their feet and ankles and to inform the clinician immediately if any are detected (WOCN, 2022; Baranoski & Ayello, 2020).
CASE (continued)
Mr. Hernandez is having his monthly follow-up appointment at the clinic. Since his last appointment, the case manager has spoken with Mr. Hernandez, who agreed to having a translator present so his wife will be fully aware of what is going on. Today, Mr. Hernandez will meet with the nurse practitioner (Celia) and physical therapist (Royce).
Mr. Hernandez is happy to share his good news with the team: he has lost 6 pounds since his last visit, which he attributes to the fact that he has stopped drinking sodas. The team congratulates him on his success as well as on the slight drop in his daily blood sugar readings. It is obvious from his demeanor that losing weight has had a positive impact on Mr. Hernandez’s attitude toward his condition, and Mrs. Hernandez asks what she can do to help her husband lose even more weight.
Celia reviews his family history with Mr. Hernandez and asks for more detail about his mother’s kidney disease. As Celia suspected, his mother had been a diabetic for many years prior to developing kidney disease. She explores with Mr. Hernandez what this meant to him, and he admitted that he had a deep fear the same would happen to him. His mother’s diabetes was poorly controlled, and he believed this was the same for all patients with diabetes. Both Celia and Royce, the PT, spend time explaining to Mr. Hernandez and his wife (via the translator) that diabetes can be controlled.
Mr. Hernandez agrees to a foot exam. A Weinstein-Semmes monofilament exam indicates that Mr. Hernandez has diabetic peripheral neuropathy in both feet, with his left foot more severely affected. There is also a noticeable deformity of his left foot, with increased temperature around the dorsal surface and ankle areas compared to the contralateral limb and increased redness on the plantar surface of the left foot along the base of the metatarsal heads. Mr. Hernandez states that it’s “impossible” for him to take time off from work and that, “although my left foot is a bit red, it feels fine.”
An X-ray ordered by Celia is normal, but the team is concerned that Mr. Hernandez is developing early-onset Charcot osteoarthropathy. When Mr. Hernandez entered the exam room, Royce noted a slight deviation in his gait that propels him more to his left side. Royce examines the patient’s footwear, which are strong leather work boots with a reinforced toe area. The toe-box is sufficiently deep to accommodate the foot, but the insole is hard and rigid from excessive wear. As a roofer, Mr. Hernandez states that this is the only type of footwear he is allowed to wear at work.
Mr. Hernandez has signs of early Charcot foot, which is a serious complication. Given that he cannot take time off from work, the team must develop suitable strategies to protect his feet from further damage. Ideally, he should be non-weight-bearing to his left foot, but since this is not a practical solution, the next best alternative is to schedule an immediate consultation with an orthotist. The physical therapist believes there is sufficient room in Mr. Hernandez’s boots for a specially fitted insole to help relieve pressure. The physical therapist also gives Mr. Hernandez a written list of recommendations to use when he is not at work, including wearing seamless socks and cushioned slippers and resting with his feet elevated.
His nurse practitioner and physical therapist must also respond to Mr. Hernandez’s statement that his left foot “feels fine.” Like many other patients with diabetes, Mr. Hernandez finds it hard to believe there could be anything seriously wrong with his foot since he feels no pain. The results of the monofilament testing are explained carefully to the patient. Celia tells him that he feels no pain because he has lost sensation to several parts of his left foot; but he is probably not aware of this because other areas of his foot still have sensation. Techniques on preventing injury to his feet, including a daily foot exam, need to be taught to Mr. Hernandez and his wife (if he agrees) and reinforced at every follow-up visit. They will need written educational materials in both English and Spanish.
In response to Mrs. Hernandez’s question about helping her husband continue to lose weight, the team believes a good place for her to start is with a review of their food intake. They reinforce the positive results she has noticed from her husband’s lower weight and his willingness to continue this effort. The case manager is consulted, who indicates that she will identify community resources where Mrs. Hernandez can learn about meal planning and healthy cooking for a person with diabetes.
As the appointment ends, Celia arranges to see Mr. Hernandez back in the clinic in two weeks. During that time, Royce will work closely with the orthotist on securing pressure-relieving customizations to Mr. Hernandez’s existing footwear.
(continues)
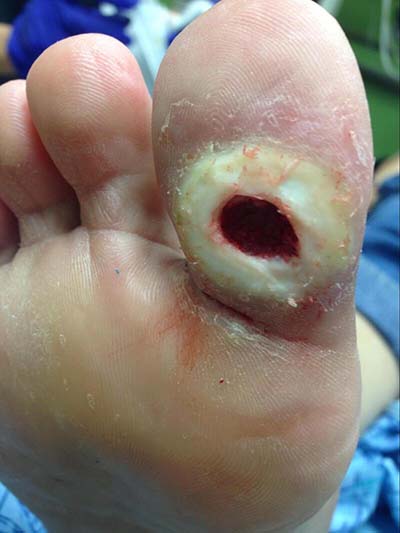
Diabetic foot ulcer with periwound callous formation. (Source: Mark A. Dreyer, Creative Commons Attribution 4.0.)