ASSESSMENT, SCREENING, AND DIAGNOSIS
Chest pain is the most common symptom of CAD. When this occurs, it is vital for the healthcare worker to assess this very carefully with a detailed description of the pain. History is an equally vital part of understanding every aspect of CAD: history of different occurrences of chest pain, medical history, social history, and family history. Laboratory work, ECGs, and imaging or radiography are the most common ways used to diagnose CAD and how severe the disease may be.
Chief Complaint: Chest Pain
Chest discomfort is a key identifying symptom of coronary artery disease, particularly in men. When a man with coronary artery disease comes to the office, clinic, or hospital with heart symptoms, the typical chief complaint is chest discomfort. Most often, the patient does not describe this discomfort as pain but instead as heaviness, pressure, squeezing, smothering, or a burning sensation.
By contrast, a woman with coronary artery disease is more likely to complain of symptoms such as nausea or abdominal discomfort; neck, throat, or jaw pain; shortness of breath; or weakness or fatigue rather than the more classic symptom of chest pain. Coronary ischemia should therefore be considered in women who appear to be acutely ill even if they do not complain of chest pain (Sole et al., 2021).
ANGINAL PAIN
Chest discomfort or chest pain can originate from many places other than the heart, but the characteristic pain of angina almost always points to ischemia of heart muscles. The pain may be retrosternal, left pectoral, or epigastric.
Classic symptoms associated with angina include:
- Chest pain or discomfort
- Pain in arms, neck, jaw, shoulder, or back accompanying chest pain
- Nausea
- Fatigue
- Shortness of breath
- Sweating
- Dizziness
(Johns Hopkins, 2021)
Onset and Provocation
Anginal pain is caused when the myocardium receives insufficient oxygen. Most activities have predictable oxygen requirements, and with stable angina the patient gets chest discomfort at predictable levels of activity that subside with rest. With unstable angina, people get chest discomfort at rest and at unpredictable times that is unrelieved by rest or medications.
Any situation that increases heart rate can trigger angina in people with coronary artery disease. Exercise is a classic cause of anginal pain: hurrying, walking up an incline, walking against a strong cold wind, working with the arms extended above the shoulders, and sexual activity are all exercises that can produce ischemic heart pain. Strong emotions or nightmares stimulate the heart through the sympathetic nervous system, and these too can cause angina.
In the case of stable angina, although the amount of exertion needed to produce chest pain is predictable, the threshold for angina will vary during the day and with the weather and temperature. After a heavy meal, for example, blood flow is diverted to the gastrointestinal organs from the heart and brain, and less exertion than usual can cause angina. Lying down changes the dynamics of blood flow, and some people get angina when they get in bed at night. Women with chronic stable angina are more likely than men to get chest discomfort when they are resting or sleeping or when they are in stressful situations.
Other medical conditions can precipitate angina in a person with coronary artery disease. Anemia, systemic infections, pneumonia, or atrial fibrillation change the balance between the heart’s need for oxygen and the available supply.
Time Course
During assessment it is essential to determine the duration of anginal pain to establish the nature of the cause. As previously stated, the chest discomfort of stable angina typically lasts from 1–5 minutes and rarely persists for as long as 10 minutes. The angina begins dully and then fades away as the patient stops and rests. Nitroglycerin tablets or sprays will usually end or lessen stable angina in a few minutes or less.
Unstable angina lasts for more than 10 minutes, and with myocardial infarctions, the pain can last for hours if untreated. When rest does not relieve classic anginal pain, then it is more likely that the patient has an acute coronary syndrome such as unstable angina or an MI.
Quality
The quality or sensation of angina has a special character. Rather than saying “pain,” patients most often use words such as squeezing, tightening, constricting, pressing, or strangling, or they clench their fists to describe the feeling of heart ischemia. They may say that they feel like there is “a band across my chest,” “a heavy weight in the center of my chest,” or “a vise that is tightening my chest.”
Location
When asked, “Where do you get this uncomfortable feeling?” patients with angina usually put a hand or fist over their sternum in the middle of their chests and say “Inside here!,” meaning retrosternally. When asked, “Does this discomfort extend anywhere else?” angina patients will often say that the feeling extends to the left shoulder, to the inside (ulnar) half of either or both arms, to the neck and jaw, or sometimes to the middle of the upper back. Additionally, women with angina may complain of pain or discomfort in the abdominal area (Lewis et al., 2020).
The pain or discomfort of angina is broad, and patients do not point to it with a finger, saying “It’s right here.” Also, patients rarely feel angina above the jaw, below the umbilicus, in the lower right chest, or localized below the left nipple. Moreover, the examiner usually cannot reproduce the pain by pushing gently on the skin or the chest wall.
| One commonly used method for quickly assessing the patient with chest pain is referred to as PQRST, mimicking electrocardiography waves. | ||
| (Lewis et al., 2020; Sole et al., 2021) | ||
| P | Provocation/precipitating events | Provocation/precipitating events What events or activities precipitated the pain or discomfort (e.g., argument, exercise)? |
|---|---|---|
| Q | Quality of pain | What does the pain or discomfort feel like (e.g., pressure, dull, aching, tight, squeezing, heaviness)? |
| R | Region/radiation of pain | Can you point to where the pain or discomfort is? Does the pain or discomfort radiate to other areas (e.g., back, neck, arms, jaw, shoulder, elbow)? |
| S | Severity of pain | On a scale of 0–10, with 0 indicating no pain and 10 being the most severe pain you can imagine, what number would you give the pain or discomfort? |
| T | Timing/treatment | When did the pain or discomfort begin? Has it changed since that time? Have you had pain or discomfort like this before? |
History
In addition to a description of individual occurrences of angina, the overall history of these episodes is important. Chronic stable angina gives predictable episodes of chest discomfort over many months, although the exact pattern of the episodes differs from patient to patient. In some patients, episodes of chest pain may occur several times a day. In others, there may be symptom-free intervals of weeks, months, or years. Occasionally, anginal attacks gradually decrease or disappear if adequate collateral coronary circulation (i.e., growth of new blood vessels) develops; this does not mean that the disease has gone away.
In contrast, acute coronary syndromes give unpredictable or steadily worsening episodes of ischemic symptoms. As acute coronary syndromes are developing, the symptoms may change from being occasional to happening constantly. An MI may give prolonged severe chest discomfort and continuous fatigue.
The chest discomfort of chronic stable angina is predictable for a given patient. Therefore, any changes in the pattern or the intensity of angina should be considered serious (Lewis et al., 2020).
NON-CAD CAUSES OF CHEST PAIN
Chest discomfort is a classic symptom of myocardial ischemia. It is also a key symptom of other medical problems, the most common of which are gastroesophageal diseases.
| Origin | Causes |
|---|---|
| (Lewis et al., 2020) | |
| Cardiovascular |
|
| Pulmonary/chest trauma |
|
| Musculoskeletal |
|
| Gastrointestinal |
|
| Infectious |
|
| Neurologic |
|
Other Symptoms of CAD
In addition to chest pain, other symptoms are frequently caused by myocardial ischemia. These symptoms include:
- Shortness of breath, especially when it feels localized to the middle of the chest
- Weakness and tiredness
- Faintness or dizziness
These three symptoms are especially common in older (age >75 years) patients and in patients with diabetes when they have episodes of heart ischemia.
Other general symptoms that may accompany or replace angina are:
- No chest pain, but discomfort in the shoulders, inside (ulnar side) of the left arm, neck, or lower jaw
- Indigestion or nausea
When accompanying angina, certain additional symptoms signal potential emergencies. For example, chest pain with fatigue, sweating, and nausea or vomiting suggests myocardial infarction.
WOMEN AND MYOCARDIAL ISCHEMIA SYMPTOMS
Healthcare professionals should be alert to the fact that women are more likely than men to present with the following as the primary symptoms of an MI:
- Dyspnea
- Gastrointestinal complaints (nausea and vomiting)
- Back pain or pressure
- Jaw pain
- Shortness of breath
- Fatigue
Women also are more likely to attribute cardiac symptoms to other causes (such as the flu, stress, and normal aging) and may delay reporting symptoms (Lewis et al., 2020).
SILENT HEART ATTACKS
Not all patients with myocardial ischemia have symptoms. Angina is a very common indicator of myocardial ischemia, and the characteristics described above are frequent and typical. Patients with all forms of CAD can have atypical feelings of chest discomfort or anginal equivalents. Moreover, ischemia severe enough to cause myocardial infarctions can occur without any chest pain, giving what are called silent heart attacks (asymptomatic myocardial ischemia). Often, the MI is discovered by ECG when the patient is seen for other problems.
CASE
Celia Brown is a 62-year-old female with a family history of CAD and a previous history of smoking 1 pack per day for 20 years (i.e., 20 pack years). Celia has arrived at the emergency department with complaints of anxiety, dizziness, weakness, and ongoing fatigue.
The nurse on duty is Robert, who questions Mrs. Brown about her current medications, which include Zocor, Atenolol, and Xanax. As Robert continues to triage Mrs. Brown, he is initially concerned that she may be having an anxiety attack since she also seems short of breath and is perspiring. He asks probing questions about any pain that she is noticing. Mrs. Brown reports that she has been having some pain in her jaw and thought it was a tooth problem and that she has had more heartburn lately and felt nauseous with cold sweats.
She tells Robert that she called her doctor with her concerns a week ago and was instructed to continue to take her Xanax and start taking an over-the-counter antacid to help with the heartburn symptoms. Mrs. Brown states that “she originally thought she might have the flu, but now she knows that there is something wrong, as she has had the symptoms for a couple of weeks.”
Although this patient has a few classic symptoms of an anxiety attack, Robert also recognizes that Mrs. Brown may be having cardiac symptoms, as women with angina do not always present with obvious complaints of chest pain. Robert discusses the case with the ED physician. Together, they continue to question Mrs. Brown about her symptoms. When asked if she had tried anything to relieve her symptoms, the patient stated that she has been taking Tylenol for the jaw pain and antacids for the heartburn symptoms.
The ED team proceeds with a cardiac evaluation along with other testing in order to rule out myocardial ischemia. Mrs. Brown’s evaluation shows that she is experiencing anginal pain. She is admitted for continuing monitoring and medical management.
Patient History
PAST MEDICAL HISTORY
The past medical history of patients with CAD may suggest that they have or are at high risk for atherosclerosis. The primary elements in a person’s medical history that should alert the clinician to the possibility of an increased risk for atherosclerotic CAD include:
- Hyperlipidemia
- Hypertension
- Diabetes
- Metabolic syndrome
- Family history of CAD
- Tobacco use
- Fatty diet
- Male gender
- Advanced age
- Lack of physical activity
- Obesity
Past medical history should also include any previous hospitalizations, any drug or food allergies, and a psychosocial history.
Ischemia
When taking a medical history, the healthcare provider may find that atherosclerosis of the coronary arteries has already revealed itself. A patient with CAD may already have had episodes of heart ischemia, such as myocardial infarctions. (See above section regarding analysis of angina.)
Peripheral Artery Disease
Atherosclerosis is a whole-body disease. Patients with coronary artery disease will often have indications of atherosclerosis in arteries outside the heart. For example, they may have a history of intermittent claudication (a result of atherosclerosis in the leg arteries), strokes or transient ischemic attacks (results of atherosclerosis in the carotid arteries), or abdominal aortic aneurysms.
Lipid Abnormalities
High levels of blood lipids predispose a patient to atherosclerosis. High levels of LDL cholesterol or low levels of HDL cholesterol can cause atherosclerosis. A patient with CAD will likely already have a diagnosis of high cholesterol.
Hypertension
High blood pressure is another major risk factor for developing atherosclerosis. The stress of an elevated BP causes endothelial injury to the arteries that increases the rate of atherosclerosis. Atherosclerosis causes narrowed, thickened arterial walls and decreases elasticity of vessels. More force is needed to pump blood through diseased coronary arteries. This increased force causes a higher BP. This added workload results in left ventricular (LV) hypertrophy and decreased stroke volume with each contraction. A patient with CAD may already be taking antihypertensive medicines (Lewis et al., 2020).
Diabetes
Diabetes puts a patient at high risk of developing CAD. People with diabetes experience CAD at an earlier age than the general population. Diabetes, especially type 2, tends to increase the level of blood cholesterol and to worsen atherosclerosis. People with diabetes have an increased tendency for endothelial dysfunction that may also increase the production of fatty streaks in the arterial walls. They also have abnormal lipid metabolism, causing high cholesterol and triglyceride levels.
People with diabetes, even those with a well-controlled glucose level, are 2 to 4 times more likely to have CAD than people without diabetes. Diabetes is more prevalent in minority populations, with African Americans at 13.3% prevalence, Hispanics at 10.2%, Asian Americans at 11.2%, and Whites at 9.4%.
The progressive increase in diabetes in the U.S. population contributes to the concurrent increase in CAD. Both diseases are also related to the aging U.S. population. Patients who have undiagnosed or poorly controlled diabetes are at highest risk. The diabetes is often diagnosed for the first time when the person has an MI (Virani et al., 2021; Lewis et al., 2020).
Metabolic Syndrome
Metabolic syndrome is the name for a cluster of health conditions that are frequently found together. The core problems are central obesity and insulin resistance. The diagnosis of metabolic syndrome can be made when the patient exhibits three of the following risk factors:
- Elevated fasting glucose
- Low HDL
- Elevated triglyceride level
- Large waist measurement
- Hypertension
Having metabolic syndrome increases a patient’s risk of developing type 2 diabetes and puts a person at high risk of developing serious atherosclerotic vascular disease with coronary artery blockage (Virani et al., 2021; Lewis et al. 2020).
FAMILY HISTORY
Patients are much more likely to develop coronary artery disease if they inherit a genetic propensity for the disease. When assessing a patient for CAD, a good indicator of this propensity is the existence of first-degree (parents, siblings, and offspring) relatives who have had an acute coronary syndrome, such as a heart attack, at an early age. For men, this would be when they were younger than 45 years, and for women, it would be when they were younger than 55 years.
There may also be a familial history of environmental or behavioral risk factors (e.g., tobacco use or alcohol abuse) or genetic risk factors (e.g., obesity, diabetes, or hypertension) that may contribute to the increased possibility of the occurrence of CAD within a family.
Similarly, racial and ethnic groups share a large percentage of their genetic variables within the group. This may explain why some racial/ethnic groups have a higher incidence of CAD than others. The higher incidence may also partly be attributable to other characteristics within the group related to dietary or other health practices (Virani et al., 2021).
SOCIAL HISTORY
Two features of a patient’s lifestyle may put them at high risk for developing CAD: smoking or other tobacco use and a high-fat diet. Assessment should include taking a careful history of current or previous smoking as well as asking about dietary habits.
Smoking one or more packs of cigarettes a day for several years doubles a person’s chance of dying from CAD. A person who stops smoking can reduce this extra risk. The lungs will clear themselves of damage caused by smoking over the course of several years. Likewise, a diet high in cholesterol, saturated fats, and trans fats increases a patient’s chances of developing artery problems from atherosclerosis, while low-fat diets or diets containing only polyunsaturated fats may reduce the risk. Unlike stopping smoking, a change in diet will not necessarily clear the arteries of the deposits of plaque (Virani et al., 2021).
PACK-YEARS
The specific measurement of smoking taken in a medical history is called pack-years. The history taker asks the number of packs of cigarettes smoked per day, on the average, multiplied by the number of years the patient has been smoking. This is based on a commercial pack of cigarettes containing 20 cigarettes, regardless of the brand or tar/nicotine content. For example, a person who smokes 2 packs per day for 30 years would be documented as having a 60 pack-year history.
Physical Exam Components
A patient with CAD who presents to the emergency department with serious cardiac symptoms can show many abnormalities on physical examination, whereas a patient with CAD who comes to the clinic or office for a regular check-up may have only a few signs of the underlying disease. During a routine physical examination, the following findings would fit with a diagnosis of CAD.
WEIGHT
Body mass index (BMI) takes weight and height into consideration by including overall body size rather than a single indicator. BMI is calculated as the weight in pounds, divided by the height in inches squared, with this figure multiplied by 703. Below are the accepted weight parameters measured in BMI:
| BMI | Status |
|---|---|
| <18.5 | Underweight |
| 18.5–24.9 | Normal weight |
| 25.0–29.9 | Overweight |
| ≥30 | Obese |
Patients with excess intra-abdominal or visceral fat (an “apple-shaped” build) are more likely to have atherosclerotic cardiovascular disease. Waist circumference is a good measure of intra-abdominal fat content: a waist circumference of >102 cm (>40 inches) in men or >89 cm (>35 inches) in women is considered in the high-risk range for cardiovascular disease (Mayo Clinic, 2021c).
VITAL SIGNS
During a routine office visit, the pulse may have a normal rate and rhythm in a patient with CAD. Tachycardia (a heartrate of >100 beats per minute) is common, however, when a patient is suffering from an episode of myocardial ischemia as a result of the stress hormones released. Bradycardia (a heartrate of <60 beats per minute) during an acute coronary syndrome can be an ominous sign because of the drop in cardiac output.
During a physical examination during a routine office visit or in a medical facility, the clinician can recommend aerobic exercises to improve physical and cardiac fitness and increase collateral circulation. The goal of aerobic exercise is to increase the resting heartrate to 50%–75% of the maximum heart rate appropriate for the patient’s age. The following chart indicates a target heart rate zone based on the age of adult patients:
| Age | Target (beats per minute) |
Maximum (beats per minute) |
|---|---|---|
| (Mayo Clinic, 2021d) | ||
| 25 | 98–146 | 195 |
| 35 | 93–138 | 185 |
| 45 | 88–131 | 175 |
| 55 | 83–123 | 165 |
| 65 | 78–116 | 155 |
Patients with CAD often have hypertension (BP ≥135/85), and the higher the blood pressure, the greater the risk of heart disease. Hypotension during a myocardial infarction is also an ominous sign because of the possible increased damage to the myocardial issue secondary to the higher afterload.
The respiration rate is usually normal (12–20 breaths per minute) in a routine office visit, but patients will breathe more rapidly under the stress of heart ischemia secondary to stress hormones.
SKIN
No unusual sweating is expected on a routine office visit, but acute coronary syndromes, especially myocardial infarctions, are often accompanied by profuse sweating (diaphoresis). The skin will also show signs of hypoxia with cyanosis, pallor, mottling, and an increase in the occurrence of decubitus ulcers and other skin lesions that do not heal readily.
HEAD AND NECK
The blood vessels of the retina may show the effects of hypertension or atherosclerosis (i.e., widened light reflections from the arteries, copper- or silver-colored arteries, white sheaths along the arteries, venous tapering or “nicking” at arterial-venous crossings, hemorrhages, or papilledema). Diabetes, which worsens CAD, produces a characteristic retinopathy.
Atherosclerotic plaques can produce local blood turbulence, which will sometimes cause a bruit that can be heard when listening to the carotid arteries. CAD that evolves to heart failure may cause jugular vein distension due to congested blood vessels.
Hypoxia may affect the individual’s ability to think or reason, orientation, and level of consciousness. Carotid arteries blocked by atherosclerotic plaques may cause confusion, hallucinations, irritability, memory loss, restlessness, pupil response, and reduced muscle strength.
THORAX
The pain of heart ischemia is usually diffuse and “somewhere inside.” If a patient’s chest pain can be reproduced by the examiner pressing on some point along the chest wall, the pain is unlikely to be angina. (In some patients with myocardial infarctions, however, broad regions of the chest become tender.)
On a routine exam, the lungs of a patient with CAD can be clear and unremarkable. With myocardial infarction, on the other hand, the patient may be breathing rapidly and may complain of shortness of breath. When ischemia has brought on some degree of heart failure, valve dysfunction, or dysrhythmia, patients can have fluid in their lungs, and crackles or coarse breath sounds may be auscultated. Chest expansion may be asymmetrical due to guarding while breathing, secondary to pain.
A routine physical exam of a patient with CAD may find no overt heart problems. If the patient has a history of ischemic episodes, there may be several adverse findings. Previous heart surgeries will have left chest scars. Hypertension or heart failure may have caused cardiomegaly. Murmurs suggest valve or papillary muscle damage, and gallops suggest heart wall damage (Sole et al., 2021).
An ischemic heart is more susceptible to dysrhythmias. Infarction causes necrotic tissue that is electrically and chemically unstable.
ABDOMEN
CAD is a risk factor for aortic aneurysms. A patient with an aortic aneurysm is at higher risk for rupture in the case of a patient with CAD with epithelial inflammation and ruptured atherosclerotic plaques in the arterial wall. An already weakened vessel wall, because of the aneurysm, is more vulnerable to internal rupture in the case of CAD (Hołda et al., 2020). Bruits from other major abdominal arteries, such as the renal arteries, can be due to atherosclerosis.
EXTREMETIES
Peripheral edema may be secondary to heart failure due to chronic ischemic heart disease. Atherosclerosis can cause weakened peripheral pulses. Diabetes can produce neuropathies, which show up as a decrease in the patient’s ability to sense stimuli in the feet with paradoxical, concurrent extremity pain.
GENITOURINARY
When CAD has progressed to a concurrent heart failure, fluid balance is an essential aspect of the physical health examination. In addition to the peripheral edema noted above, sluggish circulation may cause compromised renal function secondary to decreased renal perfusion. Retained fluid as evidenced by peripheral edema and decreased urinary output may cause an increase in blood pressure that will place more stress on arterial wall atherosclerotic plaques, promoting rupture. Compromised circulation may contribute to reduced libido in women and erectile dysfunction in men in CAD, as in diabetes (Sole et al., 2021).
Laboratory Studies
A patient being evaluated for CAD is given several laboratory tests. Certain tests are especially helpful in assessing a patient’s risk of serious heart damage from atherosclerosis. These include blood tests of lipid levels, complete blood count, fasting glucose levels, A1C, creatinine and other metabolic levels, and the possible presence of cardiac markers, which are indicators of recent heart cell damage.
BLOOD LIPIDS
High serum cholesterol levels markedly increase a patient’s risk for developing atherosclerosis-induced heart injury. The LDL (“bad”) fraction of cholesterol is the specific culprit. Patients with CAD often have one or more lipid levels in the unhealthy range.
Certain desired lipid levels have been increased somewhat in recent years with new research. The box below shows both healthy and unhealthy fasting blood lipid levels for patients with no evidence of CAD and little or no risk factors.
| Lipid | Optimal Level (in mg/dL) |
Unhealthy Level (in mg/dL) |
|---|---|---|
| Total cholesterol | <200 | >240 |
| HDL cholesterol | ≥60 | <40 (men) <50 (women) |
| LDL cholesterol | <160 | >160 |
| Triglycerides | <190 | >200 |
In the presence of CAD or significant risk factors, some target lipid levels are recommended to be even lower:
- LDL <100 mg/dL
- Triglycerides <130 mg/dL
(Sole et al., 2021)
FASTING PLASMA GLUCOSE
Patients with diabetes have a higher than normal chance of developing CAD. Diabetes will manifest as a fasting plasma glucose level of ≥100 mg/dL when measured on at least two different days. Tight control of the serum glucose will help to prevent the development of CAD when added to a program of a low-fat, low-carbohydrate diet and physical activity (Virani et al., 2021).
BLOOD UREA NITROGEN/SERUM CREATININE
Renal disease worsens atherosclerosis. The levels of BUN and creatinine in a patient’s blood can be used to screen for several kidney problems. When the CAD is complicated by heart failure, the kidneys are more likely to fail or will fail more quickly.
CARDIAC MARKERS
When myocardium is damaged, intracellular molecules leak into the bloodstream. After a myocardial infarction, specific heart proteins (cardiac markers) can be detected in a patient’s blood within hours and then for many days afterward. The standard cardiac markers are the cardiac troponin molecules. Other commonly measured proteins are the creatinine kinase molecules. Cardiac markers are used for diagnosing and following emergency cardiac events and are not measured at routine checkups for CAD.
| Marker | Normal Level | Duration of Elevation after MI |
|---|---|---|
| (Sole et al., 2021; Pagana et al., 2017) | ||
| Creatinine kinase (CK-2-MB) |
0.3–4.9 ng/mL |
|
| Myoglobin | <72 ng/mL in men <58 ng/mL in women |
|
| Cardiac troponin I (cTnI) Cardiac troponin T (cTnT) |
<0.03 ng/mL <0.1 ng/mL |
|
Creatinine kinase-2 (CK-2) was formerly the definitive diagnostic test for diagnosis of an MI. This has been replaced by serum troponin levels, as there are no other reasons for an elevation in this blood test than an MI. The troponin levels remain elevated for so much longer than the CK-2, it is possible to discover an MI has occurred when the patient presents for diagnosis after a delay of even several days.
A study of 31,492 cardiac patients used a high-sensitivity cardiac marker as a serum troponin 1 level, instead of the traditionally used troponin level, to rule out myocardial infarctions sooner. This resulted in 3.3-hour reduction in length of stay in the ED and a 59% decrease in hospital admissions. In a one-year follow-up of these patients, mortality was 1.7% compared to 2.9% in the studied patients who were tested using the traditional serum troponin levels (Anand et al., 2021).
Serum myoglobin elevation is indicative of inflammation. It is not a specific determinant of an MI, but the early release may allow the practitioner an early diagnosis.
SERUM ELECTROLYTES
Electrolytes are closely associated with cardiac contractility and conduction. Small changes from the normal serum levels in either direction may cause dysrhythmias, particularly with potassium. The following are normal serum levels of the most significant electrolytes and their panic values:
| Electrolyte | Normal Range | Panic Value |
|---|---|---|
| (Sole et al., 2021) | ||
| Potassium | 3.5–5.0 mEq/L | <2.5 or >6.6 mEq/L |
| Calcium | 8.5–10.2 mg/dL | <7.0 or >12.0 mg/dL |
| Magnesium | 1.7–2.2 mg/dL | <0.5 or >3.0 mg/dL |
Electrocardiogram
Twelve-lead electrocardiography (ECG) is the standard method for identifying dysrhythmias and conduction problems. In terms of CAD, the ECG is a quick, accurate, and noninvasive way to detect myocardial injury, ischemia, pericarditis, pulmonary diseases, left ventricular hypertrophy, and the presence of prior myocardial infarction. In the presence of any chest pain, this diagnostic test will be performed before any others.
An MI changes the electrical properties of a region of heart muscle, and these changes can be seen in the ECG. The location of the ischemic heart region can often be identified by the segments of the wave pattern that have changed. The segments of the electrical wave pattern produced during a heartbeat have been named, and changes in the ST segment and the T wave are the clearest indicators of a myocardial infarction (Sole et al., 2021).
About one quarter of patients with stable angina will have a normal ECG wave pattern when they are resting. To determine the degree of heart ischemia that a patient with chronic stable angina suffers when the heart is stressed, an ECG can be taken while the patient exercises, typically walking on a treadmill or pedaling a bicycle. Not all patients with CAD show ECG changes during such “stress testing” (see below).
Stress Testing
Stress testing is a noninvasive procedure that directly assesses the ability of a patient’s heart to cope with exercise. A stress test is a controlled way to increase the myocardial oxygen demand to find the threshold beyond which coronary arteries supply insufficient blood, causing myocardial ischemia. The lower the threshold (i.e., the smaller the stress) at which symptoms appear, the worse the patient’s coronary artery disease (Lewis et al., 2020).
Stress tests can confirm that a patient’s complaint of chest discomfort is actually anginal pain. The tests can also establish the level of activity that brings on chest discomfort. Subsequent stress tests can objectively monitor both the progression of the CAD and the efficacy of treatments.
EXERCISE STRESS TESTING
The preferred heart stressor is graded exercise, which consists of having the patient walk on a treadmill or ride on a stationary bike, progressively increasing the speed and inclination of the device to cause exercise stress while the resident is connected to a cardiac monitor, an automatic blood pressure cuff, and a pulse oximeter. This allows the examiner to monitor and record continuous data about heart rate, dysrhythmias, blood pressure, and capillary oxygen levels while the heart undergoes physical stress.
As part of exercise stress testing, patients are instructed as follows:
- To withhold certain medications prior to the test (e.g., beta blockers may limit the patient’s ability to increase heart rate during the test)
- To wear comfortable clothing and shoes for the test
- To report any level of discomfort or other symptoms that occur during testing
- About procedures that will take place before, during, and after the test
- To immediately report any chest pain, leg pain, shortness of breath, or fatigue during the testing
- That the patient will return to their hospital room or to home as soon as their heart rate and rhythm return to their pre–stress test levels
(Sole et al., 2021)
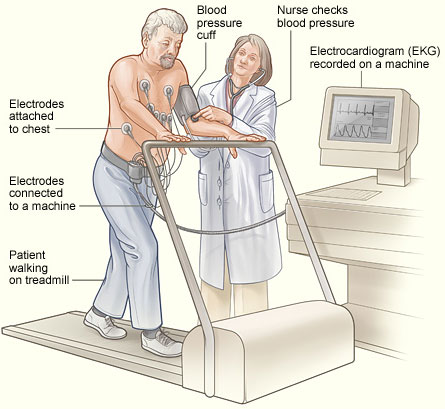
Stress testing uses graded exercise in a supervised session to assess the heart’s response to increases in its workload. (Source: NHLBI.)
During a stress test, symptoms of heart problems—such as angina, shortness of breath, severe fatigue, lightheadedness, or fainting—usually appear when patients go beyond their tolerated exercise threshold. At the same threshold, signs of heart problems—such as gallops, dysrhythmias, hypotension, inappropriate increases or decreases in heart rate, dyspnea, pulmonary rales, or cyanosis—also appear.
In addition to watching for these symptoms and signs of cardiac problems, the stress test examiner uses more objective monitoring. The typical objective monitor is electrocardiography (ECG), which shows the rate and rhythm of the heart’s electrical wave pattern, and echocardiography, which follows changes in the heart’s anatomy during exercise.
ECG stress testing is most useful in the following clinical scenarios:
- Trying to make a diagnosis of CAD in an unclear case
- Measuring the exercise limitations imposed by a patient’s CAD
Some ECG stress tests give false positives, so the test is not recommended for routine examinations of people who are not likely to have CAD. This most commonly occurs in patients who experience an increase in blood pressure while performing the stress test. The blood pressure subsequently returns to normal when the patient is in the resting phase after stress testing and is not related to the appearance of chest pain. Some ECG stress tests give false negatives, and an ECG stress test that appears normal cannot be used to discard an otherwise convincing diagnosis of CAD based on symptoms, history, and risk factors (Lewis et al., 2020).
ANSWERING PATIENT QUESTIONS
Q:What is a stress test?
A:In a stress test, a person exercises in a safe place to see how well their heart handles increased activity. Usually, you walk on a treadmill or pedal a bicycle while a physician watches your pulse rate, blood pressure, and electrocardiogram (ECG).
You will probably be asked to come to the hospital in comfortable clothes and soft shoes. When you arrive in the exercise room, electrode pads will be stuck to the skin of your chest and the wires will be attached to an ECG machine, which records the electrical activity of your heart.
Then you will exercise—slowly at first, and gradually harder. Your heart rate will get faster, your blood pressure will go up, and you will breathe more heavily. Meanwhile, the physician will keep an eye on the electrical activity of the heart. If you feel any heart symptoms, the test will be stopped. The goal is to measure exactly how much work (stress) your heart can cope with and, if your heart has difficulty, what specific heart problem may be occurring.
PHARMACOLOGIC STRESS TESTING
When patients cannot tolerate exercise, their heart can be stressed with a vasodilator drug in a monitored and controlled setting. This is done in conjunction with radionuclide scintigraphy and/or echocardiography. A physician is present at all stress tests because of the possibility of induced cardiac pain or life-threatening dysrhythmias, and the tests are tailored to the individual patient’s health. Patients who are unable to undergo strenuous exercise or are incapacitated (e.g., leg fracture) and unable to run on a treadmill or ride a stationary bike may be good candidates for this type of stress testing.
Dipyridamole (Persantine) or dobutamine (increasing heart rate and contractility) is administered intravenously. Regadenoson or adenosine may be given to cause vasodilation of normal coronary arteries. Depending on the patient’s history, thallium or sestamibi (a radioactive tracer) may also be administered with the stress test. The drugs will stimulate the heart to react as if the patient were exercising.
A partially or completely blocked coronary artery will be unable to dilate under these conditions, and this will be visible as hypoperfusion on radionuclide scanning or as hypokinesis (poor movement) on echocardiography (Sole et al., 2021).
The tracer drugs travel through the bloodstream to the heart, where they are picked up by the muscle cells. The areas of the heart that lack adequate blood supply pick up the tracer very slowly or not at all. Baseline images are compared with images taken 3 to 4 hours later. A cardiologist will determine if areas of the heart have suffered permanent damage from a previous ischemia.
Imaging Tests
Images of the heart and the coronary arteries can be obtained in a variety of ways. The least invasive techniques are chest X-rays and echocardiograms. Another technique, coronary arteriography, produces excellent views of the coronary arteries, but it is an invasive procedure, using arterial catheters, with potentially hazardous side effects.
CHEST X-RAYS
A chest X-ray is usually performed in the anteroposterior (AP) and lateral views and shows the size and shape of the heart and the condition of the lungs. Patients with CAD can have normal chest X-rays, and usually chest X-rays do not help to diagnose CAD. Sometimes, chest films will show consequences of the disease, such as cardiomegaly, cardiac positioning, cardiac abnormalities, aortic aneurysms, aortic dissections, or fluid infiltrating the pulmonary or pericardial spaces.
CORONARY ARTERY CALCIUM (CAC)
CAC is a newer type of diagnostic X-ray that scans the heart to measure the amount of calcified plaque in the coronary arteries. This noninvasive procedure can provide an early diagnosis of CAD, possibly in asymptomatic patients. This gives care providers the opportunity to initiate early treatment for prophyllaxis, such as one aspirin tablet per day or starting a regime of statins or other anticholesterol/antilipid medications.
Results of the CAC can also be used for risk stratification (putting patients into groups of similar complexity and care needs). CAC is considered a more accurate predictor of risk than the Framingham Risk Score (FRS) and the Pooled Cohort Equations (PCE), which have been found to underestimate the cardiovascular disease risk in women.
CAC can be used as an easy way to diagnose those with subclinical coronary artery plaques. Most recently, CAC is used to predict risk in borderline or intermediate levels of heart disease, particularly among people at higher risk such as those with a history of preeclampsia and early menopause (Lakshmanan et al., 2021).
ECHOCARDIOGRAPHY
An echocardiogram uses noninvasive ultrasound technology to show the size and thickness of the atria and ventricles of the heart. It also shows the heart valves in action. Used during stress testing for CAD, echocardiography can indicate which heart walls or valves are most affected by ischemic episodes. Echocardiographic stress tests are not recommended as screening tools, but many physicians use these tests to confirm a clinical diagnosis of CAD in unclear cases. Echocardiography can be used to measure ejection fraction, which is the amount of blood ejected from the left ventricle during systole. (Normal ejection fraction ranges from 55% to 70%.)
Transesophageal echocardiography (TEE) takes an ultrasonic image from a view below the heart. It is performed by inserting a flexible gastroscope into the esophagus. TEE is specifically used to view prosthetic heart valves, mitral valve function, aortic dissection, vegetative endocarditis, tumors, and emboli. The patient is NPO for 6 to 8 hours before the procedure and until the gag reflex returns post procedure. Side effects include sore throat, dysphagia, neck and shoulder pain, and a rare occurrence of esophageal perforation.
CORONARY ARTERIOGRAPHY
Coronary arteriography (also called coronary angiography or cardiac catheterization) is an invasive procedure that uses X-rays to follow dye injected into the heart or the coronary arteries. This is used to measure pressures in the ventricles, measure cardiac output, and quantify coronary arterial patency by displaying the velocity of blood flow as the dye passes through the arteries. Coronary arteriography gives as definitive a diagnosis of arterial narrowing and blockage as is possible without major surgery.
The high cost, mortality rate (>0.5%), and morbidity rate (5%) limit its use as a routine diagnostic tool. The procedure holds a high possibility of hemorrhage when the catheter through which the dye is injected is removed. Coronary arteriography is most often used in CAD patients when preparing them for possible bypass grafts or other heart operations. Coronary arteriography is also used when other tests cannot determine the cause of debilitating cardiac symptoms of ischemia (Oulette, 2020).
CORONARY COMPUTERIZED TOMOGRAPHY ANGIOGRAPHY (CCTA)
CCTA is a noninvasive form of imaging that provides an accurate diagnosis of obstructive and nonobstructive (<50% occlusion in a coronary artery) disease, risk stratification, and early initiation of appropriate treatment. This diagnostic method has proved particularly effective for use in women in spite of their smaller epicardial blood vessels.
In the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain), results showed that when CCTA is used on women, there is more effective prognostic results than with stress testing (exercise electrocardiography, exercise imaging, or pharmacologic imaging). There is also less radiation produced.
Radiation exposure is particularly a concern with the proximity of diagnostic imaging to breast tissue. Initially, there were concerns with the use of CCTA for women and the amount of exposure. Advances in technology have significantly reduced the amount of radiation from CCTA, particularly compared to other myocardial perfusion imaging techniques such as positron emission tomography (PET) and single-photon emission computerized tomography (SPECT) (Lakshmanan et al., 2021).
OTHER STUDIES
Computed tomography (CT) scanning is another common imaging tool in coronary artery disease. Coronary calcium scoring involves administration of a cardiac CT scan to collect information about the presence, location, and extent of calcified plaque in the coronary arteries. Because calcium is a marker for CAD, the coronary calcium score (a number reflecting the degree and extent of calcium deposits in the walls of the coronary arteries) can be a useful prognostic tool in coronary artery disease (Lewis et al., 2020).
Cardiac magnetic resonance imaging (MRI) is a noninvasive diagnostic test used to evaluate tissues, structures, and blood flow. It is used to diagnose CAD, aortic aneurysms, congenital heart disease, left ventricular function, tumors, blood clots, and pericardial disorders. Advantages are that the patient is not exposed to ionizing radiation and that dye can be injected to enhance results. Disadvantages are that implanted metal (e.g., pacemakers, defibrillators, or cochlear implants) may prohibit this test being performed, as the powerful magnet involved may pull too strongly on the metal, dislodging it. Another disadvantage may be that an enclosed MRI chamber can trigger claustrophobia in the patient (Lewis et al., 2020).
Magnetic resonance angiography (MRA) uses MRI technology combined with injected contrast dye to check for areas of narrowing or blockages in the coronary arteries. This technology is not as precise as coronary arteriography.