DIAGNOSING ASTHMA
There is no “gold standard” or standardized diagnostic criteria for asthma. Diagnosis is based on the patient’s history, physical examination, consideration of other diagnoses, and documentation of variable expiratory airflow limitation as determined by spirometry. In some instances, observing a response to treatment may confirm an asthma diagnosis, but lack of a response to treatment does not rule out asthma (NACA, 2021).
History
Given the wide variation in presentation, a detailed history is needed to understand each individual’s particular asthma variant. Information necessary for assistance in making a diagnosis of asthma requires asking the patient about:
- Current symptoms: Are they occurring mostly in the daytime, at night, or both?
- Pattern of symptoms: What is their course over a day, week, or year?
- Chronology: What is the chronological history of the symptoms over the person’s lifetime?
- Smoking history: Does or did the patient smoke (tobacco, cannabis)? Has the person been exposed to secondhand smoke?
- Precipitating or aggravating factors: What brings the symptoms on or what makes them worse, e.g., do they occur with exercise or with viral infections?
- Relieving factors: Is there anything that relieves the symptoms, e.g., medications?
- Home or work exposure: What types of environmental exposures are present in the home or workplace?
- Impact of symptoms: How do the symptoms affect the person’s occupation, lifestyle, and activities of daily living?
- Past history: Is there a history of allergies, including atopic dermatitis (eczema) or allergic rhinitis (hay fever)? Is there a history of other pertinent medical conditions such as COPD?
- Family history: Is there is history of asthma and/or allergies among family members?
(NACA, 2021)
EXACERBATION HISTORY
The exacerbation history is important with respect to the following:
- Usual prodromal signs or symptoms
- Rapidity of onset
- Associated illnesses
- Number in the last year
- Need for emergency department visits, hospitalizations, ICU admissions, and intubations
- Missed days from work or school
- Activity limitation
(Morris, 2020)
HISTORY OF SYMPTOMS IN CHILDREN
When taking a history of a child with wheezing or asthma-like symptoms:
- Confirm that the breathing sounds described by the parents (caregivers) as wheezing are actually “wheeze.”
- If possible, see the child during a bout of wheezing.
- Ask the parents (caregivers) to make an audio or video recording of noisy breathing.
- Ask the parents (caregivers) to describe exactly what they see or hear, and then show them a video of true wheezing and ask whether the signs match those of the child.
- Ask about the appearance of the child’s chest during episodes of noisy breathing (use of accessory muscles to breathe, retractions of the chest wall adjacent to the ribs).
(NACA, 2021)
TYPICAL ASTHMA SYMPTOM PATTERNS
Although asthma is described as a disease with episodic attacks, the pattern of clinical symptoms varies from person to person. In the medical history, recognition of the pattern of symptoms is pivotal to raising the possibility of a diagnosis of asthma. The symptom pattern of the individual should be described, noting these features:
- Whether the symptoms occur continually, episodically, or both
- Onset of symptoms, duration, and frequency
- Whether the symptoms occur perennially, seasonally, or both
- Diurnal variations, especially nocturnal and upon awakening early in the morning
- For women, whether the symptoms occur during a particular part of the menstrual cycle
Children with chronic asthma may have one of several distinct patterns of symptoms, and the asthma pattern can change over time. These may include:
- Intermittent asthma attacks with no symptoms between them
- Chronic symptoms with intermittent worsening
- Attacks that become more severe or frequent over time
- Morning “dipping” (when the symptoms worsen in the morning and improve throughout the day)
(Sawicki & Haver, 2019)
ASTHMA TRIGGERS AND AGGRAVATING FACTORS
Once asthma is acquired, it is a disease of episodic bouts of wheezing, coughing, and difficulty breathing. Regardless of the factors that entered into the initial development of asthma, there are multiple triggers and aggravators that can bring about exacerbations in a patient with an established diagnosis of asthma.
Many of the factors that are implicated in the development of asthma can also trigger an exacerbation. For asthma patients, it is necessary to learn what those triggers and aggravators are. For some, the triggers are hard to identify, and their asthma symptoms seem to appear spontaneously.
| Type | Examples |
|---|---|
| (AAFA, 2019a) | |
| Infections and comorbid conditions |
|
| Hormonal changes |
|
| Inhaled substances |
|
| Ingested substances |
|
| Physical factors |
|
| Comorbid conditions |
|
| Characteristics of the home |
|
| Environmental changes |
|
| Exercise |
|
| Emotional situations |
|
CHRONOLOGY OF THE PATIENT’S ASTHMA
The chronology section of a patient’s asthma history includes major disease events and treatments:
- First appearance of symptoms
- Date of diagnosis
- Dates of ED visits and hospitalizations (noting any ICU admissions or intubations)
- Dates of related medical and health problems
- Treatment history
- Treatment routine currently in effect
Of great significance is a past near-fatal asthma exacerbation requiring endotracheal intubation, which is the greatest single predictor of death from bronchial asthma (Chakraborty & Basnet, 2021).
CASE
Asthma History
James, age 61, recently moved to another town and paid his first visit to a new primary care physician. As part of his intake assessment, the nurse in the office collected the following information:
- Born 1950 to parents who smoked
- 1952–1955, some wheezing with colds
- 1958, mild hay fever began yearly
- 1964, started smoking (infrequently)
- 1968, smoking regularly, with occasional coughing spells
- 1972, choking/coughing episode, possible asthma diagnosed in ED
- 1972, given inhaler for asthma attacks, stopped smoking
- 1973–1979, used inhaler occasionally
- 1979, divorced, moved to new city, began smoking again
- 1980, two visits to ED for asthma attacks
- 1981–1985, physician changed PRN bronchodilator to Isoprel; slowly stopped smoking completely
- Current regimen, Proventil PRN, which is effective at reversing the 4–5 asthma episodes each year, most often in the early summer (hay fever season), and on occasion, in cold wintery weather; weight at pre-1995 levels; no ED visits in more than 20 years
FAMILY HISTORY
The family history section of the medical history lists those close relatives with atopic illnesses such as:
- Asthma
- Allergies
- Sinusitis
- Rhinitis
- Eczema
- Nasal polyps (a condition associated with asthma)
Atopic illnesses share an underlying problem with the immune system, predisposing persons toward the development of allergic hypersensitivity reactions (Morris, 2020).
SOCIAL HISTORY
Social history includes:
- Smoking
- Workplace or school characteristics
- Educational level
- Employment
- Social support
- Factors that may contribute to nonadherence of asthma medications
- Illicit drug use
(Morris, 2020)
Impact of Asthma on Patient and Family
It is always important to deal with diseases in a way that solves practical problems in patients’ lives. The goal of this section of the medical history is to elicit the practical difficulties that are posed by the patient’s asthma. It includes:
- Ways asthma symptoms disrupt the patient’s normal routine, such as the number of unplanned health visits (urgent care, ED, or hospitalization) and the number of days missed from school or work
- Limitations imposed by asthma, such as activities that cannot be undertaken and frequency of sleep disturbances
- Effect on growth, development, behavior, and school or work performance
- Issues related to impact on the family’s finances
- School characteristics that may interfere with adherence to treatment
Perception of the Disease by Patient and Family
As with all those who have chronic diseases, individuals with asthma must be the day-to-day managers of their medical care. This section of the history asks about:
- Patient’s, parents’, and spouse/partner’s knowledge of the disease process, belief in the chronicity of asthma and efficacy of treatment
- Patient’s perception and beliefs regarding the use and long-term effects of medications
- Ability of the patient, parents, spouse/partner to cope with the disease
- Level of family support and the patient’s, parents’, spouse/partner’s capacity to recognize the severity of exacerbation
- Whether the patient and family can realistically carry out the current management plan
- Whether the current plan is economically affordable
(NACA, 2021)
CASE
Patient History
Deborah is a 24-year-old teacher’s aide who works in an elementary school. She has come to her healthcare provider’s office complaining of a chest cold that she has had for two weeks and that does not seem to be getting better. She complains of frequent bouts of coughing and bringing up thick, sticky mucus. She also says she has had some occasional wheezing and difficulty breathing. Her sleep has been disturbed at least three nights a week since this all started.
Following a physical examination, she is referred to the office nurse for a complete asthma assessment. The nurse has Deborah fill out an asthma screening questionnaire. Her responses indicate a family history of asthma, a personal history of allergies, worsening of coughing and wheezing during periods of humid weather and poor air quality, more frequent episodes of sleep disturbances over the past two months, and a cigarette smoking habit (which she indicates she is trying to quit).
When asked about her work situation, Deborah notes that in addition to using a blackboard and chalk during the school day and “magic markers” to grade students’ papers, she is regularly exposed to first- and second-graders who come to school with coughs and colds. She adds that the school is located in an inner-city, low-income neighborhood not far from a factory with smokestacks that spew out thick, black smoke.
Following review of the assessment with her healthcare provider, Deborah is referred for lung function testing, and the results confirm a diagnosis of asthma.
Physical Examination
The physical examination of a patient with suspected asthma includes looking for specific evidence of atopy or allergic rhinitis:
- Eye exam
- Conjunctival congestion (redness, swelling, inflammation) or discharge
- Skin around the eyes, looking for signs of atopy or allergy:
- “Allergic” or “ocular shiners” (dark circles under the eyes caused by congestion of the nose and sinuses)
- Dennie’s lines (folds of skin below the lower eyelid caused by edema in atopic dermatitis)
- Nose interior and exterior
- Swollen or boggy normal structures (turbinates)
- Pale and violet-colored nasal mucosa due to allergic rhinitis
- Amount, color, and consistency of any nasal discharge
- Abnormal structures such as nasal polyps
- Foreign bodies (e.g., button, bead)
- Transverse nasal crease (“allergic salute”) across the lower third of the nose caused by constant upward wiping of the nose due to allergic rhinitis
- Oral cavity
- Postnasal drip (amount, color, consistency)
- Signs of inflammation of the throat
- Chest
- Shape and movement
- Increased anteroposterior diameter of chest in children due to hyperinflation, causing an abdominal breathing pattern
- Use of accessory muscles to breathe
- Prolonged expirations, inhalation period shorter than exhalation
- Lungs
- High-pitched musical wheezes most commonly heard during auscultation on expiration, crackles (rales), congestion, unequal breath sounds or any other abnormal sounds
- Prolonged end-expiratory wheeze (in a child who is not sick, forced exhalation may reveal expiratory wheezing)
- Hyperresonance found during percussion, related to trapped air in the lungs
(Morris, 2020)
EXAMINATION DURING A MILD ASTHMA ATTACK
During a mild attack, patients may:
- Be breathless after physical activity such as walking into the office
- Still be able to speak in sentences and lie flat
- Be agitated
- Have increased respiratory rate, but accessory muscles are not required to breathe
- Have a pulse rate below 100 beats per minute (bpm)
- Have moderate wheezing detectible upon auscultation of the chest but otherwise inaudible
- Have an oxygen saturation on room air higher than 95%
EXAMINATION DURING A MODERATELY SEVERE ASTHMA ATTACK
During a moderately severe asthma attack, patients may:
- Be breathless and assume a sitting position while talking
- Have an increased respiratory rate
- Require accessory muscles to breathe
- Supraclavicular and intercostal retractions and nasal flaring in children
- Abdominal breathing in children
- Have a pulse rate between 100–120 bpm
- Exhibit plainly audible expiratory wheezing
- Exhibit pulsus paradoxus (an exaggerated fall in systolic blood pressure during inspiration) of 10 to 20 mmHg
- Have an oxygen saturation on room air between 91% and 95%
EXAMINATION DURING A SEVERE ASTHMA ATTACK
During a severe asthma attack, patients may:
- Be breathless during rest, sit upright, talk in words rather than sentences, and usually be agitated
- Have a respiratory rate often greater than 30 per minute
- Require accessory muscles to breathe
- Have no interest in feeding
- Commonly exhibit suprasternal retractions
- Have a pulse greater than 120 bpm
- Exhibit pulsus paradoxus (an exaggerated fall in systolic blood pressure during inspiration) of 20 to 40 mmHg
- Exhibit loud expiratory and inspiratory wheezing
- Have an oxygen saturation on room air below 91%
- Assume a tripod position (sitting hunched over with hands supporting the torso) as severity increases
EXAMINATION DURING IMMINENT RESPIRATORY ARREST
When children are in imminent respiratory arrest, in addition to the symptoms mentioned above, they are drowsy and confused; but adolescents may not have these symptoms until they are in frank respiratory failure.
When a patient is experiencing status asthmaticus with imminent respiratory arrest, paradoxical thoracoabdominal movements in which the abdomen moves out with expirations occur. Wheezing may be absent due to severe airway obstruction, and severe hypoxemia presents with bradycardia. Pulsus paradoxus previously noted may now be absent, and this indicates respiratory muscle fatigue.
As the attack becomes more severe, there may be profuse diaphoresis along with a rise in pCO2 and hypoventilation. During such a severe episode, patients may struggle to breathe and become confused and agitated. They may try to remove their oxygen mask, complaining that they cannot breathe. These are all indications of life-threatening hypoxia (decreased oxygen reaching the tissues). As the CO2 level increases, breathing slows, the patient becomes somnolent, and there may be profuse diaphoresis. At this point almost no breath sounds may be heard and the patient is now willing to lie flat (Morris, 2020; Sharma, 2021).
PHYSICAL EXAMINATION OF CHILDREN
The physical examination of children is identical to that of the adolescent and adult, however, one of the most important goals is to identify signs and symptoms that suggest an alternative diagnosis requiring further investigation. These include:
- Upper airway diseases:
- Allergic rhinitis and sinusitis
- Obstructions involving large airways:
- Foreign body in trachea or bronchus
- Vocal cord dysfunction
- Vascular rings or laryngeal webs
- Laryngotracheomalacia, tracheal stenosis, or bronchostenosis
- Enlarge lymph nodes or tumor
- Obstructions involving small airways:
- Viral bronchiolitis or obliterative bronchiolitis
- Cystic fibrosis
- Bronchopulmonary dysplasia
- Heart disease
- Other causes:
- Recurrent cough not due to asthma
- Aspiration from swallowing mechanism dysfunction or gastroesophageal reflux
(AIM, 2021)
Diagnostic Testing
PULMONARY FUNCTION TESTS
The best objective measures of asthma are pulmonary (lung) function tests, which can quantify the degree of a patient’s airflow obstruction.
Spirometry is the most common pulmonary function test, employing a spirometer to measure the amount of air a patient can inhale completely and exhale completely as well as the rate of airflow through the airways. Spirometry assesses the unified mechanical function of the lung, chest wall, and respiratory muscles by measuring the total volume of air exhaled from a full lung, called the total lung capacity (TLC).
Spirometry is used to establish baseline lung function, evaluate dyspnea, detect pulmonary disease, monitor effects of therapies used to treat respiratory disease, evaluate respiratory impairment or disability, evaluate operative risk, and perform surveillance for occupational-related lung disease.
During the process, the technician applies a clamp to the patient’s nose to keep it shut. The patient is asked to inhale deeply and blow out as hard, as quickly, and as long as possible into the mouthpiece of the spirometer. The patient should exhale for at least 6 seconds, and at the end of the forced exhalation, the patient should again inhale as fully and as rapidly as possible. The forced vital capacity (FVC) should then be compared with the inhaled volume to verify that the forced expiratory maneuver did start from full inflation.
Before-and-after tests can also be used to monitor the effectiveness of various medications on a patient. Spirometric measurements before and two to four weeks after the patient begins a new drug can document the degree of improvement.
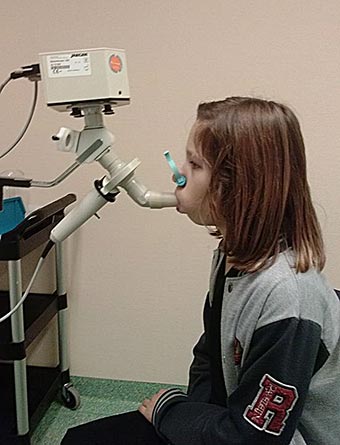
Child undergoing spirometry testing. (Source: 50Martin50, CC SA 3.0.)
For asthma, three basic lung characteristics are of clinical value:
- Forced vital capacity (FVC) is the total amount of air that can be forced quickly from the lungs after a complete inhalation.
- Forced expiratory volume in 1 second (FEV1) is the amount of air expired in the first second of forced exhalation. Airway obstruction is the most common cause of reduction in FEV1.
- FEV1:FVC ratio assesses airflow obstruction. When people with airway obstruction exhale, it takes longer than normal to empty the lungs. The amount of air expelled in one second, therefore, is reduced. The value of FEV1:FVC ratio goes down when a patient’s airways are narrowed.
(McCarthy, 2020)
Peak expiratory flow (PEF) meters are recommended for monitoring asthma in the home. PEF meters are inexpensive, handheld devices that record the maximum flow of air while a patient is forcefully emptying their lungs. Normal PEF values can vary according to a person’s sex, age, height, and race.
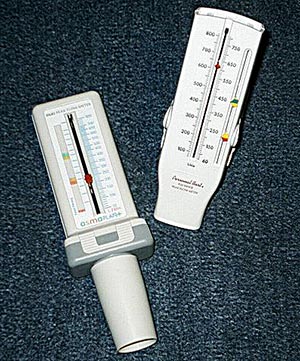
Peak flow meters. (Source: Hosseman.)
USING A PEAK FLOW METER
When using a peak flow meter, the patient:
- Measures peak flow close to the same time each day
- Makes certain the sliding marker or arrow on the meter is at the bottom of the numbered scale before beginning
- Stands straight, takes in a complete breath, closes the lips tightly around the mouthpiece, and blows out as hard and as fast as possible until all air is emptied from the lungs
- Writes down the number the marker or arrow has reached along the numbered scale
- Repeats the steps above two more times
- Records the highest reading of the three, which is called the patient’s predicted normal value, or “personal best”
| Zone | Measurement | Finding |
|---|---|---|
| (ALA, 2020c) | ||
| Green | 80%–100% of patient’s normal | Asthma is under control |
| Yellow | 50%–80% of patient’s normal | Rescue medicines should be used; a medical visit may be needed |
| Red | <50% of patient’s normal | Signals a medical alert; emergency care is needed |
Body plethysmography, also known as a lung volume test, measures the amount of air the person can hold in the lungs and the amount of air that remains after forced exhalation. It is used to determine whether exposure to substances in the home or workplace have caused lung damage and whether there is a need for a change in treatment (McCormack, 2020).
Gas diffusion test or diffusing capacity of the lung for carbon monoxide (DLCO) determines how well the alveoli are delivering oxygen to and removing carbon dioxide from the blood into the capillaries surrounding them. The person inhales air containing a very small amount of carbon monoxide and a tracer gas, such as methane or helium, holds the breath for 10 seconds, and then rapidly exhales. The exhaled gas is tested to determine how much of the tracer gas was absorbed during the breath. Another name for this test is lung diffusion testing (NIH, 2021a).
FRACTIONAL EXHALED NITRIC OXIDE TESTING (FeNO)
FeNO testing can help determine how much inflammation is present in the airways. It does not test directly for asthma but can assist in confirming an asthma diagnosis in adults and children ages 5 and older. It is also done if spirometry cannot be performed. Exhaled nitric oxide testing is technically complex and is not routinely used in monitoring (Cloutier et al., 2020).
CHILDREN AND LUNG FUNCTION TESTING
Most children who have asthma develop their first symptoms before 5 years of age. Asthma in children ages 0–5 years, however, can be hard to diagnose since spirometry is not usually done in children younger than 6 years because of the difficulties in obtaining reliable forced expiratory maneuvers in this age group.
Alternative measurements that require less patient cooperation have been developed for use in young children; however, they are not widely available. These alternatives include:
- Interrupter technique (Rint) measures airway resistance, including lung airways and rig cage during tidal breathing (normal, restful breathing). The method is based on transient interruption of airflow at the mouth for a brief period during which alveolar pressure and mouth pressure reach equilibrium, and the pressure change at the mouth can be used to calibrate the resistant of the airways.
- Forced oscillation technique (FOT) and impulse oscillometry system (IOS) are both widely used in pediatric settings. They are noninvasive techniques performed during tidal breathing, requiring only minimal cooperation from the patient. FOT and IOS assess bronchial hyperresponsiveness by employing small-amplitude pressure oscillations superimposed on normal breathing.
- Multiple breath washout (MBW), also referred to as gas dilution, measures the clearance of a gas from the lungs. It is performed during tidal breathing, during which a marker gas, usually helium, less frequently nitrogen, is washed out with 100% oxygen. Preschool children perform the test in a seated position, and a video can be used for distraction and to promote a regular breathing pattern.
- Single-breath counting (SBC) is a novel technique for measuring pulmonary function in children. SBC is the measurement of how far the child can count using a normal speaking voice after one maximal effort inhalation. The count is in cadence to a metronome that is set at two beats per second. SBC has been found to correlate well with standard measures of pulmonary function.
(Rosen & Colin, 2020; Morris, 2020)
BRONCHODILATOR TESTING AND BRONCHIAL PROVOCATION
Among the features of asthma that vary from individual to individual is the innate degree of hypersensitivity of the patient’s airways. In some patients, a small amount of irritation triggers a severe reaction. Other patients, however, are less sensitive and get much less bronchoconstriction with the same amount of irritation.
Bronchodilator testing is recommended in almost all adult and adolescent patients with airflow limitation on their baseline spirometry. Acute reversibility of airflow obstruction is tested by administering two to four puffs of a quick-acting bronchodilator (e.g., albuterol) using a valved holding chamber (spacer) and repeating spirometry 10 to 20 minutes later. Measurements can also be made before and after administration of a nebulized bronchodilator.
A positive bronchodilator response, however, is not sufficient to make the diagnosis of asthma, as the response may be seen with other conditions such as COPD. Asthma is normally distinguished from these other conditions by the capacity for a large increase in FEV1. The definition of a “large response,” however, is not standardized.
Bronchoprovocation is useful for diagnosing asthma in patients with normal baseline airflow. It can be used to identify or exclude airway hyperresponsiveness in patients with an atypical presentation or isolated symptoms of asthma, especially cough. Several types of bronchoprovocation testing are available to assess airway responsiveness in specific patient situations. These may include:
- Pharmacologic drugs to cause narrowing of the airways, including methacholine or mannitol
- Exercise challenge
- Eucapnic voluntary hyperpnea, which mimics the effect that prolonged exercise has on the airway
- Food additive challenge
- Antigen challenge
During this test, a direct provocative stimulus is used to stimulate bronchoconstriction. People with asthma are more sensitive to such stimuli than those without asthma. Although these challenges are very safe, they may make the patient feel dizzy or uncomfortable and can cause symptoms of an asthma attack.
A positive bronchoprovocation test is not entirely specific for asthma. False negative results are uncommon, and a negative test performed in a patient off of asthma controller therapy does reliably exclude the diagnosis of asthma.
Other specialized testing can be used when assessing for exercise-induced bronchoconstriction as well as evaluation for possible occupational asthma by obtaining measurements before and after a work shift (Irvin, 2020; ALA, 2021a).
ALLERGY TESTING
Many persons with asthma have atopy. In these people, allergic reactions from inhaled biologic substances will increase their sensitivity to asthmatic triggers. The best protection from this increased sensitization is for the patient to avoid inhaling the allergens, and to do this, patients must identify the allergens that cause them trouble.
As a first step in building a list of probable offending allergens, the patient keeps a diary of exposures and symptoms. The second step is allergy testing to verify or reject at least some of the suspected allergens. Ridding a patient’s environment of offending allergens can be time-consuming and expensive, and allergy testing will indicate which specific types of cleaning and avoidance should be worth the effort.
Allergy testing can be done in vivo and in vitro.
In Vivo Tests (Skin Tests)
In vivo tests are quick, fairly reliable, and cost effective. There are three common methods of allergy skin testing.
In a skin prick test (also called a puncture or scratch test), a series of tiny drops of allergens are placed on the skin, and the skin underneath each drop is pricked with a needle or lancet so that the allergen will penetrate beneath the skin surface. Several allergens can be tested at the same time. If the person has an allergy to an allergen, a dime-sized wheal will appear at the prick site and will be red and itchy. In adults, the test is usually done on the forearm, and children may be tested on the upper back.
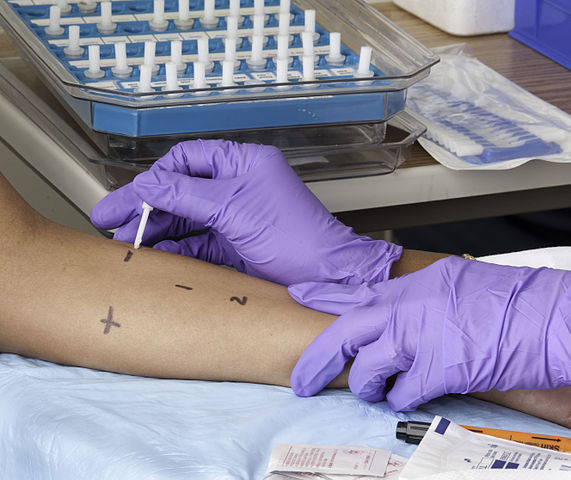
Skin prick test. (Source: National Institutes of Health.)
If the skin prick test is negative, an intradermal test may be done, in which the allergen is injected into the skin. This test is more likely to be used to find out if the patient is allergic to bee venom or a drug (e.g., penicillin). Or it may be used if the skin prick test was negative and the clinician still thinks the patient is allergic to the allergen.
Another type of skin testing is the patch test, in which an allergen is applied to a patch and placed on the skin, where it remains for 48 hours. The clinician looks at the area in 72 to 96 hours, and if the skin has become red, irritated, and itchy, the results may indicate an allergy (NIH, 2021b).
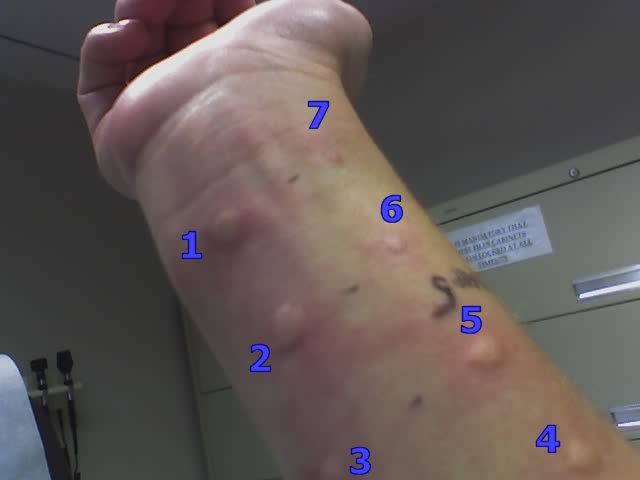
Intradermal skin testing. (Source: Wikimedia, CC SA 2.0.)
Intradermal testing carries a slightly higher risk of provoking significant allergic reactions than other methods. In rare instances a patient can have a severe, immediate allergic reaction (anaphylaxis) and require emergency management.
Intradermal tests can also be more accurate than skin prick and patch tests, but sometimes they may be falsely positive. In some cases, a person may have a positive response to a substance but have no problems with that substance in everyday life. Also, if the dose of allergen is large, even people who are not allergic to it can have a positive reaction (Mustafa, 2021; Mayo Clinic, 2020c; Bhargave, 2020).
In vivo allergy testing is not without risk. In some people, an area of swelling, redness, and itching may develop hours after the test and persist for as long as a couple of days. Other side effects might be pain or bleeding at the injection site, dizziness, or lightheadedness during testing.
In Vitro Tests
In vitro tests use a blood sample from the patient to detect circulating IgE antibodies to specific allergens.
The most commonly used in vitro tests are immunoassays, which include the enzymes-linked immunosorbent assay (ELISA or EIA) and variations on this technique (fluorescent enzyme immunoassays [FEIA] and chemiluminescent immunoassays).
A positive immunoassay test only confirms the presence of the antibody, but actual reactivity must be determined by the patient’s history or by a supervised challenge. An allergen-inhalation challenge is done in specialized centers able to handle potentially significant reactions. This test is often needed to help diagnose occupational asthma.
Skin testing is preferred over in vitro testing because it is quicker, less expensive, more sensitive, and does not require submitting a blood sample. In certain circumstances, however, in vitro testing is advantageous over skin testing because it does not pose a risk of an allergic reaction. It is used in older adults with cardiovascular disease, patients with sensitivities to allergens that are associated with severe anaphylactic reactions, and patients with histories of severe reaction to very small amounts of the allergen.
Immunoassays are valid for infants as young as 6 weeks of age and can be performed on capillary blood samples. In vitro tests may be preferable for those with skin conditions, such as widespread and severe atopic dermatitis, or dermographism (exaggerated weal and flare response occurring within minutes of skin being stroked or scratched). In vitro tests are often indicated to confirm negative skin tests. Another advantage is that in vitro tests are not affected by medications that might cause confusion with skin testing.
Immunoassays are available for:
- Foods
- Insect venoms
- Environmental allergies such as pollen, dust mites, or cockroaches
- Natural rubber latex
- Some beta-lactam drugs such as penicillin or cephalosporins
- Some occupational allergens
(Kowal & DuBuske, 2021)
LABORATORY DATA
Laboratory studies are not usually a major part of diagnosing or following asthma, but a few tests can give supportive evidence and may be used to exclude other diagnoses.
- CBC (complete blood count), to screen for eosinophilia or significant anemia and to evaluate blood cells to provide information on infection and inflammation
- Alpha-1 antitrypsin level, for lifelong nonsmokers to exclude emphysema
- Comprehensive metabolic panel, to evaluate overall body organ function, including kidney, liver, and lungs
- Sweat test or trypsin/chymotrypsin, to rule out cystic fibrosis in both children and adults
- AFB tuberculin testing, to rule out tuberculosis and nontuberculous mycobacteria
- Lung biopsy, to evaluate lung tissue for damage and for cancer
(Fanta, 2020a; Lab Tests Online, 2021)
Other laboratory tests related to asthma may include:
- Blood gases (ABGs) during severe asthma attacks, to predict respiratory failure and the consequent need for mechanical ventilation (only patients whose oxygenation is not restored to over 90% with oxygen therapy require an ABG)
- Sputum cultures, to diagnose lung infections caused by bacteria
- Sputum cytology, to assess for the increased concentration of eosinophils and neutrophils that occurs in patients with asthma
(Morris, 2020)
PULSE OXIMETRY
Pulse oximetry (PO) measures the percentage of hemoglobin that is carrying oxygen to determine hypoxemia in patients with acute asthma. PO is often used in children to grade the severity of an acute asthma exacerbation and to determine whether the child requires hospitalization.
A pulse oximeter device is placed on the finger, nose, toe, earlobe, or forehead, and a beam of light is passed through to the blood in the capillaries. The amount of oxygen in the blood is measured, along with the pulse rate.
A pulse oximetry reading should always be considered an estimate of the oxygen saturation. Therefore, numbers from a pulse oximeter should not be used in isolation. A reading may be anywhere from 2% to 4% higher or lower than the actual oxygen level in the arterial blood (ALA, 2021b).
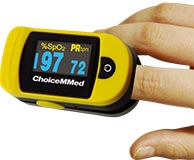
A finger-mounted pulse oximeter with pulse bar taking measurements through the fingernail. (Source: Thinkpaul, CC SA 3.0.)
FDA ALERT
In February 2021, the FDA issued an alert on limitations of pulse oximeters. The pulse oximeter may not give accurate or consistent readings in persons with poor circulation, dark skin pigmentation, thick skin, current tobacco usage, cool skin temperature, dark fingernail polish, long artificial nails, or soiled fingers (FDA, 2021a).
An oxygen saturation (SpO2) reading of 95% or higher is normal for a healthy individual. A reading of 91%–95% is clinically acceptable but low and may be due to the patient being a smoker. Readings from 70%–90% are unsafe levels and indicate hypoxemia. Less than 70% indicates extreme lack of oxygen. However, clinical correlation is always necessary since the exact cutoff below which tissue hypoxia ensues has not been defined.
Patients with chronic lung disease often have a degree of hypoxia, in which case target saturation rates generally fall between 88%–92%. These patients are often aware of what is normal for them. A drop of 3% or more below what is normal for the patient warrants further assessment, and a drop of 4% or more may require hospital admission (Yale Medicine, 2021).
IMAGING STUDIES
A chest X-ray is valuable for revealing complications or alternative causes of wheezing. It is usually more useful in the initial diagnosis of asthma than in the detection of exacerbation, although it is valuable in excluding complications, including pneumonia and asthma mimics, even during exacerbation.
In most patients with asthma, chest X-ray findings are normal or may indicate hyperinflation. Because pneumonia is one of the most common complications of asthma, chest X-ray is indicated in those with fever to rule out pneumonia.
For diagnostic purposes, atypical presentations, and hospital admissions, chest X-rays should be taken. In asthma, X-rays can show the presence of superimposed infections, atelectasis (collapse of an expanded lung), or pneumothorax (abnormal presence of air in the pleural cavity, leading to collapse of the lung). Chest films may also help to distinguish asthma from allergic bronchopulmonary aspergillosis, sarcoidosis, congestive heart failure, pulmonary emboli, foreign body aspiration, and lung cancer (Morris, 2020).
High-resolution CT (HRCT) is a second-line examination useful for patients with chronic or recurring symptoms and those with possible complications, such as allergic bronchopulmonary aspergillosis and bronchiectasis. Findings in bronchial asthma include:
- Bronchial wall thickening
- Bronchial dilatation
- Cylindrical and varicose bronchiectasis
- Reduced airway luminal area (the major determinant of airway resistance)
- Mucoid impaction of the bronchi
- Centrilobular opacities, or bronchiolar impaction
- Linear opacities
- Air trapping, as demonstrated or exacerbated with expiration
- Mosaic lung attenuation, or focal and regional areas of decreased perfusion
(Morris, 2020)
ECG
ECG is done to determine whether the heart is the cause of severe asthma symptoms, such as irregular heartbeat, an enlarged heart, or previous damage to the heart muscle. It is also done to determine if the patient’s heart is healthy enough for medications that may be prescribed for asthma management (Asthma UK, 2021b).
Patients who are severely symptomatic should have ECG monitoring. Sinus tachycardia and ECG evidence of right heart strain are common in patients with acute asthma (Morris, 2020).
NUCLEAR IMAGING
Technetium-99m DTPA radioaerosol lung scintigraphy is a classic technique that shows the extent of major airway distribution, peripheral distribution, and absorption in the oronasal air passages. Ventilation scanning has been used as an indicator of ventilation defects in asthmatic children as well as to assess distribution of aerosol and particulates from asthma medications (Morris, 2020).
24-HOUR pH PROBE
A 24-hour pH probe can be used to help diagnose GERD if a patient is not being managed effectively with asthma therapy. This is an outpatient procedure done to measure the pH or amount of acid that flows into the esophagus from the stomach during a 4-hour period (Morris, 2020).
Classifying Asthma Severity
There are two methods for classifying asthma severity:
- The Global Initiative for Asthma (GINA) Guidelines classify asthma according to treatment requirements.
- The National Asthma Education and Prevention Program (NAEPP) Guidelines classify asthma in adults based on signs and symptoms and in children based on signs, symptoms, and treatment requirements.
| Asthma Classification | Treatment Requirements |
|---|---|
| (GINA, 2021) | |
| Mild | Well-controlled with rescue medication alone or with low-dose controller treatment such as low-dose inhaled corticosteroids (ICS), leukotriene receptor antagonists, or chromones |
| Moderate | Well-controlled with low-dose inhaled corticosteroids (ICS) combined with long-acting beta-2 agonists (LABA) |
| Severe | Requires high-dose inhaled corticosteroids combined with long-acting beta-2 agonists to prevent uncontrolled asthma or asthma that remains uncontrolled despite this treatment |
| Asthma Classification | Signs, Symptoms, Treatment Requirements | |
|---|---|---|
| Children 0 to 4 Years | Adults and Children 5 Years and Above | |
| (Morris, 2020) | ||
| Intermittent (most common and least severe form of asthma) |
|
|
| Mild persistent |
|
|
| Moderate persistent |
|
|
| Severe persistent |
|
|