COMMON CATEGORIES OF MEDICAL ERRORS AND HOW TO PREVENT THEM
Errors can be placed into five general categories: surgical, diagnostic, medication, devices and equipment, and systems failures (including healthcare-associated infections, falls, and healthcare technology). Common areas in each of these categories are described below.
Surgical Errors
Wrong-site, wrong-procedure, and wrong-patient surgical errors are relatively rare. It is estimated that in the United States such errors occur in approximately 1 of 112,000 surgical procedures and are infrequent enough that an individual hospital would only experience one such incident every 5 to 10 years. However, this includes only procedures performed in an operating room. If procedures performed in other settings such as ambulatory surgery are included, the rate of error may be much higher (AHRQ, 2019d).
Retained foreign bodies (also called retained surgical items) and unintentionally retained foreign objects are defined as objects retained after skin closure following an invasive procedure. It is estimated that 1 in every 5,500 procedures involves a retained foreign body, leading to adverse outcomes, including the need for additional operations, readmission or prolonged length of stay, infections, other health risks, and even death. Most unintended retained foreign bodies are associated with failures in leadership, communication, or other human factors that should be under the control of the operating team.
The Joint Commission indicates that between 2012 and 2018, the following items were retained:
- 102 instruments
- 52 catheters and drains
- 33 needles and blades
- 30 instances of packing
- 14 implants
- 6 specimens
(Pellegrini, 2019)
The most frequent anesthesia-related adverse events include:
- Breathing circuit disconnections
- Inadvertent gas flow change
- Syringe swap
- Gas supply problem
- Intravenous apparatus disconnection
- Laryngoscope malfunction
- Premature extubation
- Breathing circuit connection error
- Hypovolemia
- Tracheal airway device position changes
(Rayan et al., 2019)
PREVENTING WRONG-SITE, WRONG-PROCEDURE, OR WRONG-PERSON SURGERIES
Surgical errors are not the sole responsibility of the operating surgeon. All operating room personnel have a role in ensuring patient safety by verifying the surgical site and pointing out a possible error.
To reduce the risk of wrong-site, wrong-procedure, or wrong-person surgeries, the Joint Commission has developed a Universal Protocol checklist that includes the concept of a surgical “timeout,” a planned pause before beginning a procedure in order to review important aspects of the procedure with all involved personnel. Although it was initially designed for operating room procedures, timeouts are now required before any invasive procedure. Comprehensive efforts to improve surgical safety have incorporated timeout principles into surgical safety checklists.
It should be noted, however, that many cases of wrong-site, wrong-procedure, or wrong-patient errors would still occur despite full adherence to the Universal Protocol. Errors may happen well before the patient reaches the operating room, a timeout may be rushed or otherwise ineffective, and production pressures may contribute to errors during the procedure itself. Preventing such errors depends on the combination of system solutions, strong teamwork, safety culture, and individual vigilance (AHRQ, 2019d).
The WHO Surgical Safety Checklist was developed after extensive consultation aimed at decreasing errors and adverse events, and increasing teamwork and communication in surgery. This checklist has gone on to show significant reduction in both morbidity and mortality and is now used by a majority of surgical providers around the world (WHO, 2021).
ELEMENTS OF THE WHO SURGICAL SAFETY CHECKLIST
A surgical checklist is an algorithmic listing of actions to be taken in any given clinical situation intended to make everyone aware that others expect these things to be done.
“SIGN IN” checklist must be completed orally and in writing before induction of anesthesia (with at least a nurse and anesthetist).
- Has the patient confirmed their identify, site, procedure, and consent?
- Is the site marked?
- Is the anesthesia machine and medication check complete?
- Is the pulse oximeter on the patient and functioning?
- Does the patient have a:
- Known allergy?
- Difficult airway or aspiration risk?
- Risk of >500 ml blood loss (7 ml/kg in children)?
“TIME OUT” checklist must be completed orally and in writing before skin incision (with nurse, anesthetist, and surgeon).
- Confirm all team members have introduced themselves by name and role
- Confirm the patient’s name, procedure, and where the incision will be made
- Has antibiotic prophylaxis been given within the last 60 minutes?
- For the anticipated critical event:
- To surgeon:
- What are the critical or nonroutine steps?
- How long will the case take?
- What is the anticipated blood loss?
- To anesthetist:
- Are there any patient-specific concerns?
- To nursing team:
- Has sterility (including indicator results) been confirmed?
- Are there equipment issues or any concerns?
- To surgeon:
- Is essential imaging displayed?
“SIGN OUT” checklist must be completed orally and in writing before the patient leaves the operating room (with nurse, anesthesia provider, and surgeon).
- Nurse verbally confirms:
- The name of the procedure
- Completion of instrument, sponge, and needle counts
- Specimen labeling (read aloud specimen labels, including patient name)
- Whether there are any equipment problems to be addressed
- To surgeon, anesthetist, and nurse:
- What are the key concerns for recovery and management of this patient?
(WHO, 2021)
PREVENTING RETAINED FOREIGN OBJECTS
Recommendations to reduce incidents of unintended retained foreign objects related to human factors include:
- Provide team training
- Address disruptive behavior
- Minimize distractions and interruptions
- Account for objects inserted in the wound
- Methodologically explore the surgical site prior to closure
- Verify integrity of objects upon removal
- Educate staff about risks of retained foreign objects and risk-reduction strategies
- Assess competency (initial and maintenance) of personnel
Recommendations for leadership issues include:
- Prioritize a culture of safety
- Conduct a proactive risk assessment and implement policies and procedures based upon it
- Celebrate successes, but also encourage reporting of near misses
Recommendations for communication issues include:
- Verbally acknowledge removal of objects
- Discuss removal of objects during standardized debriefing after procedures
- Discuss the need for packing removal during handoff
- Document verification of removal and integrity of objects
(Pellegrini, 2019)
CASE
Cheryl, a left-hand-dominant author, was scheduled for a left carpal tunnel release to alleviate her left-hand pain. Immediately prior to her being transferred to the operating room, her surgeon verified the procedure and side with her and marked the surgical site with a purpose-made surgical site marker in accordance with facility policy.
After the “time out” and induction of general anesthesia, the site was prepped and draped, the surgeon made a Z-shaped incision from the proximal phalanx of Cheryl’s left middle finger to the middle of her left palm and began to carefully dissect down through the soft tissue. The scrub, an experienced perioperative nurse, was perplexed by the placement of the incision, since the usual incision for a carpal tunnel release goes from the palm (in line with the ring finger) toward the wrist. The scrub did not say anything, since the surgeon was new to the facility, had just completed a fellowship in hand surgery, and had already performed several newly developed procedures with which the nursing personnel were not familiar.
After examining the tissue in Cheryl’s palm, the surgeon commented on the lack of thickening of the ligament in the palm and the inconsistency between his findings and her reported symptoms of ring finger pain and difficulty in doing keyboard work. At this point, both the circulating nurse and anesthesia provider stated that the proposed procedure was a carpal tunnel release. This was confirmed by the surgeon, anesthesia provider, circulating nurse, and scrub visualizing the surgical schedule and Cheryl’s chart (history and physical, surgical consent, and surgical safety checklist).
The surgeon closed the incision and made an appropriate incision for a carpal tunnel release, and the procedure was completed without further issue. After Cheryl was transported to the postanesthesia care unit (PACU), the surgeon spoke with her husband. He informed him of the incident and told him that a complete review of all that had transpired would be done that day. The surgeon later spoke to Cheryl and told her that he would give her a complete explanation the following day once all of the medications she had received were no longer affecting her understanding or memory.
The surgeon met with Cheryl and her husband and adult daughter the following day. He described the nature of the error (that a trigger finger release incision was made instead of the carpal tunnel release incision intended), how it had occurred, and what steps would be taken to improve that aspect of OR safety. The night of surgery, the family had briefly considered filing a lawsuit, but after meeting with the surgeon, they were satisfied with the full and honest disclosure of the incident and decided not to sue.
Diagnostic Errors
Diagnostic errors are common, accounting for 17% of preventable errors in hospitalized patients. A systematic review of autopsy results done over four decades found that almost 9% of the deceased patients experienced a major diagnostic error that was not detected prior to death. One in every six patients is affected by a diagnostic error, and 1 in every 1,000 primary care visits causes preventable harm.
Most diagnostic errors that occur in primary care settings include failure to order appropriate tests, faulty interpretation of data, failure to follow-up, and failure to refer. A common error is closing the diagnostic process prematurely, which can result in a common, benign diagnosis for a patient with uncommon, serious disease. Delaying treatment after the diagnosis is made is the third most common error, resulting in increased costs for readmission and further treatment (AHRQ, 2019e; Rodziewicz et al., 2021).
Making a diagnosis is a very complex process, with over 10,000 known diseases and 3,500 kinds of laboratory tests, but only a small number of symptoms, so that any one symptom may have dozens or hundreds of possible explanations. Diagnostic testing may be helpful in clarifying the issue, but it is mostly a matter of observing the clinical course, which takes time. An error can occur at any step in the diagnostic process: getting a complete patient history, doing an appropriately thorough examination, obtaining the right tests, or interpreting tests correctly (SIDM, 2021).
Cognitive psychology applied to healthcare has shown that clinicians frequently use heuristics (shortcuts or “rules of thumb”), also called cognitive bias, to come up with a provisional diagnosis, especially when faced with a patient with common symptoms. Heuristics allow people to solve problems and make judgments quickly and efficiently.
Heuristics lead to diagnosis of a current patient biased by experience with past cases, relying on initial diagnostic impression despite subsequent information to the contrary, and placing undue reliance on test results or “expert” opinion.
Clinicians are frequently unaware of diagnostic errors that they have committed, particularly if they do not have an opportunity to see how their diagnoses turned out over time. Therefore, regular feedback to clinicians on their diagnostic performance is helpful. Unfortunately, reliable decision support or feedback systems do not yet exist (AHRQ, 2019e).
Traditionally the autopsy has been the gold standard for diagnosis, but autopsy rates have progressively declined over the past few decades, and teaching institutions have not followed the recommendation to perform autopsies on 25% of inpatient deaths. As a result, clinicians are not receiving feedback on their diagnosis (AHRQ, 2019e).
HIGH-RISK DIAGNOSES
Prevention must include clinician awareness of the most commonly misdiagnosed conditions and taking extra precautions to seek and confirm the diagnosis. Clinicians must be aware of and carefully consider the following common “high-risk” diagnoses:
- Acute renal failure
- Acute pyelonephritis
- Acute vascular occlusion
- Adverse effect of medication
- Aneurysms
- Angina
- Appendicitis
- Arrhythmias
- Asthma exacerbation
- Cellulitis
- Decompensated heart failure
- Hypertension
- Metastatic cancer
- Metabolic disorders like hypoglycemia, gout
- Osteomyelitis
- Primary malignancy
- Pneumonia
- Spinal cord compression
- Symptomatic anemia
- Urinary tract infection
(Rodziewicz et al., 2021)
CASE
A serious outbreak of the Ebola virus was underway in Liberia in western Africa. A man traveled from Liberia back to his home in Texas, where he began to experience fever, nausea, and abdominal pains, prompting him to go to the emergency department (ED). There he reported to the nurse his recent travel to Liberia but denied contact with sick people. He was misdiagnosed and sent home. Days later he returned to the ED, tested positive for Ebola, began receiving care, but died soon after.
Investigation of this misdiagnosis discovered that the patient’s travel history was obtained by the nurse and entered into his electronic medical record (EMR). The patient, however, had not mentioned the fact that he had had contact with an Ebola patient prior to leaving Liberia. Additionally, the examining physician did not see the travel portion of the patient’s history because it was in the nursing section of the EMR, which physicians can, but often don’t, routinely check. Every facility makes choices about what information shows up routinely in what part of the EMR, and this hospital chose not to include the travel history in the physician section of the EMR.
Nurses are not required to inform doctors about everything they do and document. However, important information is generally personally communicated to the physician. Although the importance of this patient’s travel history should have been recognized because of the amount of publicity surrounding the Ebola outbreak at that time, the nurse did not inform the physician personally.
The nurse asked the right questions about travel, but the patient failed to disclose important information for an unknown reason. The nurse correctly entered the travel history into the medical record but failed to verbally inform the physician, and the physician chose not to read the nurse’s notes. All of these actions illustrate the importance of communication in the prevention of medical errors such as this misdiagnosis and delayed treatment.
Medication Errors
Every year in the United States, 7,000 to 9,000 people die due to a medication error. In addition, hundreds of thousands experience but often do not report an adverse reaction or other complication related to a medication. Clinicians have access to more than 10,000 prescription medications, and nearly one third of adults in the United States take five or more medications.
Each year, adverse drug events account for nearly 700,000 emergency department visits and 100,000 hospitalizations. Nearly 5% of hospitalized patients experience an adverse drug event, making them one of the most common types of inpatient errors. Ambulatory patients may experience adverse drug events at even higher rates, and transition in care is also a well-documented source of preventable harm related to medications (Tariq et al., 2021; AHRQ, 2019e).
Medication errors may be due to human errors but often result from a flawed system with inadequate backup to detect mistakes. Medication errors may occur at any step, including:
- Ordering/prescribing. The clinician must select the appropriate medication, dose, frequency, and duration.
- Transcribing. In a paper-based system, an intermediary must read and interpret the prescription correctly.
- Dispensing. The pharmacist must check for drug-drug interactions and allergies and release the appropriate quantity of the medication in the correct form.
- Administering. The correct medication must be supplied to the correct patient at the correct time, either by a nurse, other trained staff, patient, or caregiver.
- Monitoring. This includes laboratory tests, side effects, effectiveness of therapeutic action, and vital signs.
- Documenting. The name, strength, and quantity of drug; the date and time administered; and the name of the person administering the drug must be entered in the patient’s medication administration record in a timely manner.
(Tariq et al., 2021)
ERRORS IN PRESCRIBING AND TRANSCRIBING
Errors occur most commonly during the ordering/prescribing and transcribing stages, accounting for almost 50% of medication errors. Even with the increasing use of electronic health records, which has helped avert errors at the ordering and transcribing stages, such errors continue to occur.
The most common errors in the ordering/prescribing step include:
- Ordering the incorrect drug
- Ordering the incorrect dose
- Ordering the wrong interval or drug schedule
- Ordering the wrong route of administration
- Ordering the wrong infusion rate
- Ordering the wrong dose form (tabs, liquid, immediate-release instead of extended-release)
- Distortions, including illegible handwriting, misunderstood symbols, use of abbreviations, or improper translation
- Use of abbreviations
- Inappropriate use of decimal points
- Incomplete order
- Ordering and not being alerted to allergies
- Lack of awareness of known contraindications
- Ordering and not being aware of pre-existing medical conditions
- Ordering without reviewing and being aware of current medications being taken
(Tariq et al., 2021)
The National Medication Error Reporting program permits subscribing healthcare institutions to report and track medication errors and finds that medical abbreviation errors account for 4.7% of those errors reported to MedMarx. The most common medical abbreviation error was the use of “QD” (one daily), accounting for 43.1% of all errors, followed by “U” for units, “cc” for “ml,” and other decimal errors.
The most common drug abbreviation name that led to an error was the use of “MS” or “MSO4” for morphine sulfate. At least 81% of the errors were noted to occur at the time of ordering the medication, while errors at the transcribing and dispensing stage occurred at a lower frequency (Tariq & Sharma, 2020).
The Institute for Safe Medication Practices has developed a list of abbreviations that are routinely misinterpreted (see “Resources” at the end of this course).
BAD HANDWRITING
Bad handwriting by physicians has become such a major problem that the Institute of Safe Medication Practices has recommended the complete elimination of handwritten orders and prescriptions. Although the handwriting problem can be solved by using electronic records in which everything is typed, errors may still occur from entering the wrong drug, dose, or frequency (Tariq et al., 2021).
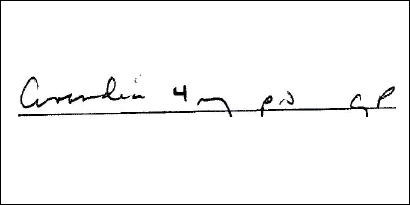
Illegible prescription: Avandia (a diabetic medication) confused with Coumadin (an anticoagulant), both available as 4 mg oral tablets.
(Source: AHRQ, 2003.)
Preventing Prescription and Transcription Errors
The first line of defense against medication errors should be the prescribing clinician, who must have all the information needed to make the best possible prescribing decisions for each patient. Such information includes evidence-based recommendations on medications for different illnesses or conditions, including correct dosing, benefits and potential risks, and accurate and complete information about the patient’s current medications, illnesses, comorbid conditions, known allergies, or past history of adverse reactions to medication.
Strategies for preventing errors when prescribing include avoiding unnecessary medications by adhering to conservative prescribing principles, which include:
- Maintaining heightened awareness concerning side effects
- Exercising skepticism about new drugs
- Remaining alert for high-risk medications
- Involving the patient in decision-making
- Considering long-term impacts of medications prescribed
- Considering patient age and body weight
- Considering liver and kidney function
Use of a computerized provider order entry (CPOE) allows clinicians to directly place orders electronically, with orders transmitted directly to the recipient. CPOE avoids the necessity for transcribing an order and thus reducing risk of error.
A third strategy is to perform a medication reconciliation at times of transitions in care, such as when transferring a patient from one facility to another or from one unit in a facility to another. Medication reconciliation refers to the process of avoiding such inadvertent inconsistencies across transitions in care by reviewing the patient’s complete medication regimen at the time of admission, transfer, or discharge and comparing it with the regimen being considered for the new setting of care. This involves reviewing each medication and comparing it against the medication administration record.
To reduce transcription errors, a double-check procedure is recommended in which another nurse on the same shift or incoming shift reviews all new orders to ascertain that each order is correctly noted and transcribed on the physician’s order and on the medication administration record. Read-back to another professional is another procedure in which a nurse reads back an order to the prescribing physician or another nurse to make certain the medication ordered is correctly transcribed (APF, 2020; Saljoughian, 2020).
ERRORS IN DISPENSING
The second line of defense against medication errors is the individual who is dispensing the medication. Dispensing medications involves preparing and packaging a prescription drug or device in a container and labeling the container with information required by state and federal law.
Dispensing errors in U.S. clinical and community pharmacies occur at an average rate of 4 in 250 prescriptions. Forty-one percent of all medication incidents related to information technology are due to choosing the wrong drug. One third of incidents are associated with confusion of similar drug names, and nearly half were associated with drug strength confusion. Errors by pharmacists are usually judgmental or mechanical.
Judgmental errors include:
- Failure to detect drug interactions
- Inadequate drug utilization review
- Inappropriate screen
- Failure to counsel the patient appropriately
- Inappropriate monitor
Mechanical errors include:
- A mistake in dispensing or preparing a prescription
- Administering an incorrect drug or dose
- Giving improper directions
- Dispensing the incorrect dose, quantity, or strength
The most common causes for dispensing errors involve:
- Workload
- Similar drug names
- Interruptions
- Lack of support staff
- Insufficient time to counsel patients
- Illegible handwriting
(Tariq et al., 2021)
Preventing Dispensing Errors
Strategies to reduce the risk of medication dispensing errors because of drug confusion include:
- Verifying the prescription entry is correct
- Clarifying any ambiguous information such as prescriptions that are illegible or use nonstandard abbreviations and other symbols
- Checking prescriptions thoroughly and verifying by another person
- Providing patient counseling
- Checking for drug-to-drug interactions and allergies
- Supervising dispensing medications by pharmacist assistants
- Opening containers and showing them to the patient (patients may raise an alert if the medication looks different from what they usually take)
- Using tall-man lettering (TML), a technique that uses uppercase lettering to highlight the differences between similar drug names by capitalizing dissimilar letters (e.g., “CISplatin” vs. “CARBOplatin”)
- Using barcode scanners to check whether the selected drug from the shelf is the same as the selected drug on the dispensing screen
(Campmans et al., 2018; FDA, 2020a)
ERRORS IN ADMINISTRATION
In the administration stage, errors include:
- Failing to follow the “five rights” to medication administration:
- Right patient
- Right drug
- Right dose
- Right route
- Right time
- Failing to educate the patient as to why the drug is being prescribed
- Leaving a medication at the bedside without knowing if it was taken
- Omitting medications
- Administering an unauthorized medication
- Not shaking a medication that should be shaken before use, leading to overdose or underdose
- Crushing medications not intended to be crushed
- Failing to follow facility policies and procedures
Most medication-related errors occur in hospital settings, where nurses administer the majority of medications. Studies have found an estimated median error rate of 8% to 25%. About one third of all medical errors causing harm to hospitalized patients occur during the medication preparation and administration phase.
The most common type of error has been found to be wrong time of administration, followed by omission and wrong dose, wrong preparation, or wrong administration rate for intravenous medications. Administration of intravenous medications has the highest error rate estimate, ranging from 48% to 53%. One study estimated a 73% probability of at least one error occurring during a single administration of intravenous medication.
Errors in medication administration can occur as a result of individual-level slips and lapses, but may also result from system-level failures such as understaffing, human factor problems, and other latent conditions. The strongest risk factors for adverse drug events are polypharmacy and hospitalized pediatric patients, since many medications must be dosed according to their weight.
Another substantial source of medication administration error includes patients and caregivers, who are responsible for the vast majority of medication administration at home. Error rates have been found to range from 2% to 33%, with dosage errors, omissions, and wrong medications the most common types of errors (AHRQ, 2019f; Hanson & Haddad, 2020; APF, 2020).
Preventing Administration Errors
In inpatient settings, interventions to prevent medication administration errors include:
- Barcoding for both medications and patients
- Adherence to the “five rights” of medication safety
- Smart infusion pumps for intravenous administration
- Single-use medication packages
- Package design features such as tall-man lettering for look-alike drug names
- Minimizing interruptions
“RIGHTS” OF MEDICATION SAFETY
Efforts have been made to increase the number of “rights” beyond the original five. However, an increasing number of recent studies have also identified inadequacies of the “five rights” in significantly removing errors due to factors that place workplace strains on staff. Workload problems, understaffing, and interruptions have all been found to make the five rights difficult to comply with all of the time. Thus, adding new “rights” to be considered and followed will likely not improve the outcome (Hanson & Haddad, 2020).
Few of these interventions are likely to be successful in isolation, and efforts to improve safe medication use must also focus on transitions to home, primary care, and patient caregiver understanding and administration of medication. These efforts include:
- Patient education
- Revised medication labels to improve patient comprehension of administration instructions
- Multicompartment medication devices for patients taking multiple medications in ambulatory or long-term care settings
(AHRQ, 2019f)
Individually, those who administer medications must also safeguard against medication errors by:
- Being proficient in medication calculations
- Maintaining up-to-date pharmacologic knowledge
- Informing patients of a medication’s therapeutic effects
- Documenting accurately once a medication has been administered
PATIENT-CENTERED CARE AND MEDICATION COMPLIANCE
Research has found medication noncompliance in 20% to 59% of elderly patients. Patients who are noncompliant tend to have multiple chronic conditions, be forgetful, and experience adverse effects from medications. Patient noncompliance may result in medication errors that can lead to hospitalization or serious injury.
Patient-centered care establishes a partnership between clinicians and patients. Joint decisions are made that incorporate patients’ wants, needs, and preferences, which result in better decision-making and active participation by patients in their own care (APF, 2020).
ERRORS IN MEDICATION MONITORING
Monitoring and assessment are essential to the process of administration of medications. Errors can occur regarding the assessment of vital signs, lab values, ability to swallow, and patient’s self-report. Monitoring involves observing the patient to determine if the medication is working, is being used appropriately, and is not harming the patient. Types of errors in monitoring that can occur include:
- Failure to monitor effectiveness of therapeutic action of a medication
- Lack of awareness of side effects of a medication
- Failure to monitor, assess, and report laboratory tests
- Failure to monitor, assess, and report vital signs
- Failure to educate patients about potential side effects
- Failure to comply with a pain management program
- Communication failures during handoff procedures to accepting nurse
(Tariq et al., 2020, AHRQ, 2020e)
ERRORS AND HIGH-ALERT MEDICATIONS
The Institute for Safe Medication Practices (ISMP) defines a high-alert medication as a drug that has a heightened risk of causing significant patient harm when used in error. Although errors may or may not be more common with such medications, the consequences of errors are much more devastating. High-alert medications are at the top of the list of drugs involved in moderate-to-severe patient outcomes when an error occurs.
The ISMP lists high-risk medications according to what is commonly used in acute care settings, community settings, and long-term settings. These lists are updated every few years based on error reports submitted to ISMP, reports of harmful errors in the literature, and input from practitioners and safety experts.
High-alert medications specific to acute care settings include:
- Adrenergic agonists, IV (e.g., epinephrine, norepinephrine)
- Adrenergic antagonists, IV (e.g., propranolol, metoprolol, labetalol)
- Anesthetic agents, general, inhaled, and IV (e.g., propofol, ketamine)
- Antiarrhythmics, IV (e.g., lidocaine, amiodarone)
- Antithrombotic agents (e.g., anticoagulants, Factor Xa inhibitors, thrombotics)
- Cardioplegic solutions
- Chemotherapeutic agents, parenteral and oral
- Dextrose, hypertonic, 20% or greater
- Dialysis solutions, peritoneal and hemodialysis
- Epidural and intrathecal medications
- Inotropic medications, IV (e.g., digoxin, milrinone)
- Insulin, subcutaneous and IV
- Liposomal forms of drugs (e.g., liposomal amphotericin B)
- Moderate sedation agents, IV (e.g., dexmedetomidine, midazolam, lorazepam)
- Moderate and minimal sedation agents, oral, for children (e.g., chloral hydrate, midazolam)
- Opioids, including IV, oral, and transdermal
- Neuromuscular blocking agents (e.g., succinylcholine)
- Parenteral nutrition preparations
- Sodium chloride for injection, hypertonic, greater than 0.9% concentration
- Sterile water for injection, inhalation, and irrigation in containers of 100 ml or more
- Sulfonylurea hypoglycemics, oral (e.g., chlorpropamide, tolbutamide)
(ISMP, 2018)
Preventing Errors with High-Alert Medications
The Institute for Safe Medication Practices makes the following recommendations for reducing errors with high-alert medications:
- Standardize the ordering, storage, preparation, and administration of these medications
- Improve access to information about these drugs
- Limit access to high-alert medications
- Use auxiliary labels and automated alerts
- Employ redundancies—duplicate devices used for backup purposes to prevent or recover from the failure of a specific part of the process (e.g., asking another nurse to perform an independent check)
- In community/ambulatory settings, it is recommended that mandatory patient education should occur
(ISMP, 2018)
FDA Warnings for High-Risk Medications
The FDA requires high-risk medications with serious or life-threatening risks be given a label referred to as a black box warning, the FDA’s strongest labeling requirement. Before adding a box warning, however, the FDA must have evidence that the drug poses a significant risk. This comes from observations and studies conducted after the drug has been on the market. Unfortunately, this means that new drugs that have just been put on the market rarely will have these warnings.
Despite these warnings, however, it has been shown there has been no associated reduction in prescribing such medications. This may be attributed to:
- Unawareness by prescribers that the FDA has issued the warnings
- Prescribers thinking that even though there is a high risk, the drugs have a superior benefit-risk ratio to alternative medications
- Prescribers thinking that the safety concern is not as severe as the warning suggests
- Prescribers choosing to continue prescribing them while using strategies to reduce risk, such as close monitoring of patients
(ISMP, 2018)
PREVENTING ADVERSE EVENTS DUE TO PATIENT-CONTROLLED ANALGESIA (PCA)
Checklists for safe use of PCA pumps are available. The Physician-Patient Alliance for Health and Safety checklist recommends certain steps be taken when initiating, refilling, or reprogramming PCA pumps, and PCA checks to be done at shift change and hourly.
PCA pump initiation, refilling, or programming a change requires:
- Assessment of the patient for increased risk of respiratory distress due to:
- Obesity
- Low body weight
- Current medication that can potentiate sedative effects
- Preexisting conditions such as asthma, COPD, and sleep apnea
- Advanced age
- Preprocedural cognitive assessment to determine the capability of the patient to participate in pain management (may not be suitable for pediatric patients)
- Provision of information to the patient on proper use of the PCA and purpose of monitoring
- Two healthcare providers independently verify the patient’s:
- Identification
- Allergies, if any
- Drug selection and concentration as prescribed
- Dose adjustment, if any
- PCA pump settings
- Line is attached to the patient and tubing is inserted into the pump
- Electronic monitoring:
- Pulse oximetry
- Capnography
Change of shift and every hour requires:
- Assessing patient for level of pain, alertness, and adequacy of ventilation
- Verifying PCA pump settings
- Verifying electronic monitoring of pulse oximetry and capnography
- Documenting patient assessment and condition, PCA dosing, and monitoring
(PPAHS, 2020)
CASE
In a large Midwestern city, a nurse working on the obstetrics unit of a local hospital was halfway through the second of two eight-hour shifts, and she asked to go home because she was tired. The hospital denied her request, stating staffing would be inadequate (fatigue and RN staffing). The nurse was assigned a young female in active labor. The patient stated that she had spoken to her doctor beforehand and had agreed to an epidural for delivery.
In order to save time (workload and time pressures), the nurse took a bag of epidural anesthesia from a storage locker without a doctor’s order, brought it to the patient’s room, and laid it on the work counter (deliberate violation of medication administration guidelines, policies, and procedures). The IV bag had a bright-red label that read “for epidural use only.”
In the meantime, an IV antibiotic was ordered and delivered to the patient’s room. The nurse picked up what she believed was the IV antibiotic (similar packaging or product) and hung it (deliberate violation of medication administration guidelines, policies, and procedures). Shortly thereafter, the patient had a seizure and died. Her infant was delivered live by cesarean section.
The investigation of the incident revealed that the nurse:
- Was fatigued and under time pressure
- Failed to follow hospital procedures requiring a doctor’s order before removing drugs from the storage locker
- Failed to recognize the bright-red intrathecal warning label on the IV bag
- Failed to follow the hospital’s policy and procedure to scan medication labels before drugs were administered
- Failed to follow the “rights” of medication administration as described in the hospital’s policy and procedure manual
Investigation further revealed that shortcuts were common practice on the unit.
Initially the nurse was charged with a felony, which was later reduced to civil charges, and her license was suspended.
Tubing Misconnections
The FDA reports that medical device misconnections can occur when one type of medical device is attached in error to another type of medical device that performs a different function. Tubing misconnections can occur for several reasons, including:
- Similar design of many connectors and the widespread use of connectors with similar shapes and in similar sizes
- Human error arising from conditions such as:
- Multiple connections for one patient
- Poor lighting
- Lack of training
- Time pressure
- Fatigue
- High-stress environment
EXAMPLES OF TUBING MISCONNECTIONS
- Enteral feeding tube connected to an IV
- Enteral feeding tube connected to ventilator-inline suction catheter
- Blood pressure cuff tubing connected to an IV port
- IV tubing connected to trach cuff
- IV tubing connected to nebulizer
- Oxygen tubing connected to a needleless IV port
- IV tubing connected to nasal cannula
- Syringe connected to trach cuff
- Epidural solution connected to a peripheral or central IV catheter
- Epidural line connected to an IV infusion
- Bladder irrigation solution utilizing primary IV tubing connected to a peripheral or central IV catheter
- Foley catheter connected to NG tube
- IV infusion connected to an indwelling urinary catheter
- IV infusion connected to an enteral feeding tube
- Primary IV tube connected to a blood product meant for transfusion
(FDA, 2017)
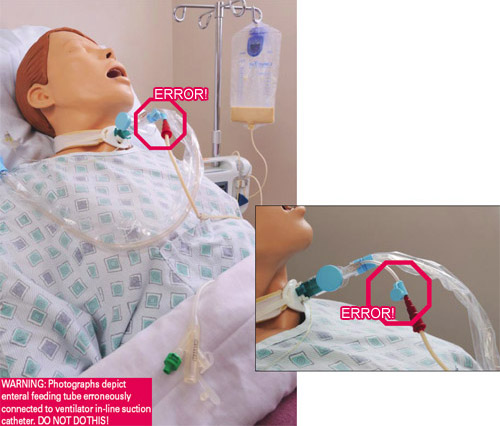
Patient’s feeding tube is incorrectly connected to the instillation port on the ventilator in-line suction catheter, delivering tube feeding into the patient’s lungs, causing death. (Source: FDA, 2017.)
PREVENTING TUBING MISCONNECTIONS
Attempts to prevent device misconnections have included color-coding, labels, tags, and training. However, these methods alone have not effectively solved the problem, because they have not been consistently applied, nor do these methods physically prevent the misconnections.
The FDA, the standards community, the International Organization for Standardization, and the medical device industry have taken actions to reduce the likelihood of medical device misconnections. These actions include the development of standardized connector designs for the specific medical applications intended to physically prevent connections with devices used for other medical applications.
In order to reduce the chances of tubing misconnections, non-Luer lock connections have been introduced. These include the NR-Fit connector for neuraxial and regional anesthesia catheters and the Enfit connectors for feeding tubes.
These connectors are designed to be incompatible with Luer adaptors, which are commonly used in IV applications. The connectors look and secure very similar to a Luer threaded lock system, although the design is larger and, therefore, incompatible with connectors for unrelated delivery systems such as trach tubes, IV lines, and catheters (Rodziewicz et al., 2021).
Until new connectors are universally adopted, the following interventions offer healthcare providers with strategies such as the use of ACT to prevent device misconnections (see table):
| Label | Step | Actions |
|---|---|---|
| (FDA, 2018) | ||
| A | Assess equipment |
|
| C | Communicate |
|
| T | Trace |
|
Risk managers, nurse managers, clinical educators, and similar personnel may:
- Inform clinicians, patients, and caregivers in home-based or ambulatory care when new devices are intended for use in the healthcare facility or at home
- Emphasize the risk of tubing and catheter misconnections in clinical staff orientation and training
(FDA, 2018)
Errors Related to Medical Devices and Equipment
The FDA regulates devices that support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential unreasonable risk of illness or injury by using a process of scientific and regulatory review to evaluate safety and effectiveness. The FDA has the following three levels of classification for medical devices and equipment:
- Class I devices do not come into contact with a patient’s internal organs, the central nervous system, or cardiovascular system. Examples include:
- Bedpan
- Tongue depressor
- Oxygen mask
- Bandages
- Class II devices are more likely to come into sustained contact with a patient, such as those that come into contact with a patient’s cardiovascular system or internal organs and diagnostic tools. Examples include:
- Catheters
- Blood pressure cuffs
- Syringes
- Blood transfusion kits
- Surgical gloves
- Class III devices usually sustain or support life, are implanted, or present a potential unreasonable risk of illness or injury. Examples include:
- Breast implants
- Pacemakers
- Defibrillators
- High-frequency ventilators
- Fetal blood sampling monitors
- Implanted prosthetics or other devices
As experience and knowledge about a device increase, the original classification of a device can be changed through reclassification. To reclassify a device, the FDA must:
- Publish a proposed order that includes a summary of valid scientific evidence that supports the reclassification
- Convene a device classification panel meeting
- Consider comments from the relevant public docket
(Rodziewicz et al., 2021; FDA, 2019)
Design flaws, misuse, and malfunction of medical devices and equipment are all common causes of medical errors. Subtle differences in a familiar pattern using a device can affect the speed and accuracy of data entry, and the lack of standardization invites user mistakes. Poor medical device design and lack of usability testing have also been repeatedly discussed as being key factors in many device-related incidents.
An increasing number of medical devices are also implanted in patients. These include complex programmable cardiac pacemakers, defibrillators, deep-brain stimulation neurotransmitters, and laser surgical devices. Any malfunction of such devices can be serious and even life threatening. Each year the FDA receives several hundred thousand reports of suspected device-associated deaths, serious injuries, and malfunctions.
Mandatory reporting of such events must be done by manufacturers, importers, and device user facilities. Healthcare professionals, patients, caregivers, and users are also encouraged to voluntarily report adverse events to MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program. User facilities must report suspected medical device-related deaths to both the FDA and the manufacturer. A user facility is not required to report a device malfunction but can voluntarily advise the FDA of such product problems. The FDA uses the reported information to monitor device performance, detect potential device-related safety issues, and contribute to benefit-risk assessments of these products (FDA, 2020c).
CASE
Jory, a 17-year-old boy, fractured his arm in several places following a tackle and fall while playing football. He was taken to the nearby hospital, where he underwent surgical repair. Postoperatively he was placed on morphine delivered via a pump. His heart rate, respirations, and blood oxygen levels were being monitored. Through the evening hours, Jory was alert, oriented, and had stable vital signs. When the night shift took over, it was ordered that the morphine should be shut off and that he should be placed on routine vital sign checks and oral pain medication.
During the night, the nurse entered his room to assess his vital signs and found that he was nonresponsive and barely breathing. It was discovered that the morphine pump, a newly acquired piece of equipment, had not been shut off but had accidently been turned to the “high” setting. Jory was lucky; he survived the overdose.
The following investigation found that the new device was designed differently than the old one, with an additional step required in the shut-off process, and the nurse had not received training in the use of the new pump.
PREVENTING ERRORS RELATED TO MEDICAL DEVICES AND EQUIPMENT
Workplaces, instruments, devices, and equipment should be designed and developed to consider human factors. A healthcare professional can maximize safety through participating in the selection process, utilizing proactive risk-assessment methods, and confirming that equipment is maintained.
Health professionals should:
- Standardize equipment, such as infusion pumps and monitors, in similar care environments
- Be involved in setting and evaluating institutional, organizational, and public policy related to technology
- Make sure that the technology used meets quality and safety standards
Institutions should:
- Make decisions concerning technology with the input of critical stakeholders (those with a financial interest, medical leaders, clinicians, patients, and vendors)
- Have policies and processes related to maintenance, training, monitoring, and reporting adverse events related to technology
(Rodziewicz et al., 2021)
Healthcare-Associated Infections (HAIs)
HAIs are infections that occur while receiving healthcare in a hospital or other healthcare facility and that first appear 48 hours or more after admission or within 30 days after having received healthcare. HAIs are considered system failures and are often preventable.
As many as 1 in 31 hospitalized patients acquire an HAI, resulting in increased complications, length, and cost of the hospital stay. AHRQ reports that HAIs are the most common complications of hospital care (CDC, 2020a).
Common types of HAIs include:
- Catheter-associated urinary tract infections (CAUTIs) occur at a rate of approximately 3% to 10% per day of catheterization, making duration of catherization an important risk factor. Complications of CAUTIs include sepsis, bacteremia, and involvement of the upper urinary tract (Fekete, 2020).
- Surgical site infections (SSIs) occur in 2% to 4% of all patients undergoing inpatient surgical procedures. Although most infections are treatable with antibiotics, SSIs remain a significant cause of morbidity and mortality after surgery. They are the leading cause of readmissions to the hospital following surgery, and approximately 3% of patients who contract an SSI will die as a result (AHRQ, 2019g).
- Central line–associated bloodstream infections (CLABSIs) are laboratory-confirmed bloodstream infections that are not secondary to an infection at another body site, due to the presence of an intravascular catheter that terminates at or close to the heart, or in one of the great vessels that is used for infusion, withdrawal of blood, or hemodynamic monitoring (NHSN, 2021). Ninety percent of all bloodstream infections are caused by central venous access devices.
- Peripheral vascular catheter (PVC)-associated bloodstream infections occur in approximately 0.18% of patients (Blauw et al., 2019).
- Clostridioides (Clostridium) difficile (C. diff) infections (CDIs) cause life-threatening diarrhea. It is usually a side-effect of taking antibiotics. Those most at risk are patients, especially older adults, who take antibiotics and also receive medical care and people staying in hospitals and nursing homes for a long period of time (CDC, 2019a).
- Hospital-acquired pneumonia (HAP) occurs 48 hours or more after hospital admission at a rate of 5 to 10 per 1,000 hospital admissions. Ventilator-associated pneumonia (VAP) is a subset of HAP occurring in intensive care units that presents more than 48 to 72 hours after tracheal intubation and is thought to affect 10% to 20% of patients receiving mechanical ventilation for more than 48 hours (Shebl & Gulick, 2020).
Many efforts to prevent HAIs have focused on acute care settings, but increasingly, healthcare delivery, including complex procedures, is being shifted to outpatient settings such as ambulatory surgical centers, end-stage renal disease facilities, and long-term care facilities. These settings often have limited capacity for oversight and infection control when compared to hospital-based settings. Patients with HAIs, including those caused by antibiotic resistant organisms, often move between various types of healthcare facilities, and prevention efforts must now expand across the continuum of care (ODPHP, 2020a).
One of the most important reasons in healthcare settings for the spread of bacteria, some of which are antibiotic-resistant and can prove life threatening, is the failure of physicians, nurses, and other caregivers to practice basic hand hygiene. Studies show that on average healthcare providers clean their hands less than half of the times they should, contributing to the spread of HAIs (CDC, 2020b).
PREVENTING CATHETER-ASSOCIATED URINARY TRACT INFECTIONS
The CDC (2019b) recommends the following actions supported by evidence-based research for preventing urinary tract infections:
- Insert catheters only for appropriate indications.
- Leave catheters in place only as long as needed.
- Avoid use of urinary catheters in patients and nursing home residents for management of incontinence.
- Avoid routinely using urinary catheters in operative patients unless necessary.
- Perform hand hygiene immediately before and after insertion or any manipulation of catheter device or site.
- Ensure that only properly trained persons insert and maintain catheters.
- In acute care hospital settings, insert catheters using aseptic technique and sterile equipment.
- In nonacute care settings, use clean technique for intermittent catheterization.
- If ultrasound bladder scanners are used, ensure that equipment is cleaned and disinfected between patients.
- Properly secure indwelling catheters after insertion to prevent movement and urethral traction.
- Unless clinically indicated, use the smallest-bore catheter possible consistent with good drainage.
- Follow aseptic insertion and maintain a closed drainage system.
- If breaks in aseptic technique, disconnection, or leakage occur, replace the catheter and collection system.
- Maintain unobstructed urine flow.
- Keep collecting bag below level of bladder at all times.
- Do not rest collecting bag on the floor.
- Empty collecting bag regularly using separate, clean container for each patient; avoid contact of spigot with the container.
- Obtain urine samples aseptically. If small amount needed, aspirate from needleless sampling port with sterile syringe/cannula adapter after cleaning the port with a disinfectant.
- If obstruction occurs and catheter material is contributing to obstruction, change the catheter.
- Comply with CDC hand hygiene recommendations and Standard Precautions.
Also consider:
- Alternatives to indwelling urinary catheterization in selected patients
- Urinary catheter systems with preconnected, sealed catheter-tubing junctions
- Use of portable ultrasound devices for assessing urine volume to reduce unnecessary catheterizations
CASE
Brenda is a nursing assistant instructor at the local technical college. Today she has taken a group of students to their clinical site, the Marshall Green Nursing Home, which has had a higher than usual number of urinary tract infections over the last several months. One of her students, Annie, is assigned to an elderly gentleman who has an indwelling urinary catheter in place. The care plan indicates he should use a bedside drainage bag during the night and a leg bag during the day. The nursing assistant assigned to the patient tells Brenda his leg bag is in the bedside stand wrapped in a towel.
When Annie locates the bag, it is in a washbasin wrapped in a towel. She finds there is no cap on the end of the tubing that is to be inserted into the catheter, and she shows this to Brenda. Annie has been taught that the end of the tubing must be protected by capping it with a sterile cap in order to maintain a closed system and to prevent bacteria from contaminating the system. Brenda approaches the nursing assistant and tells her about the lack of the cap and the risk for infection. The nursing assistant replies, “We never put a cap on the end of it.”
Brenda tells Annie to obtain a new leg drainage bag, instructing her to ensure that she cleans the end of the bedside drainage bag connection and caps it with the cap removed from the new leg-bag tubing before storing it in the bedside cabinet. She then brings the contaminated leg bag to the supervising nurse, who says she will report it and speak to the nursing assistant about it. With the help of Brenda, Annie completes an incident report.
PREVENTING SURGICAL SITE INFECTIONS
The CDC recommends the following measures for the prevention of surgical site infections (SSIs):
- Administer preoperative antimicrobial agents only when indicated by published clinical practice guidelines, and time the administration so that a bactericidal concentration is established when incision is made.
- Administer appropriate parenteral prophylactic antimicrobial agents before skin incision in all cesarean section procedures.
- In clean and clean-contaminated procedures, do not administer additional prophylactic antimicrobial agent doses after the surgical incision in closed in the OR, even in the presence of a drain.
- Do not apply antimicrobial agents (i.e., ointments, solutions, or powders) to the surgical incision with the aim of preventing SSI.
- Application of autologous platelet-rich plasma is not necessary for the prevention of SSI.
- Consider the use of triclosan-coated sutures for the prevention of SSI.
- Implement perioperative glycemic control, and use blood glucose target levels lower than 200 mg/dL in patients with and without diabetes.
- Maintain perioperative normothermia.
- Advise patients to shower or bathe the entire body with either antimicrobial or nonantimicrobial soap or an antiseptic agent on at least the night before the day of the procedure.
- Perform intraoperative skin preparation with an alcohol-based antiseptic agent unless this is contraindicated.
- Application of microbial sealant immediately after intraoperative skin preparation is not necessary for prevention of SSI.
- The use of plastic adhesive drapes with or without antimicrobial properties is not necessary.
- Consider intraoperative irrigation of deep or subcutaneous tissues with aqueous iodophor solution.
- Do not withhold transfusion of necessary blood products from surgical patients undergoing prosthetic joint arthroplasty as a means of preventing SSI.
- In clean or clean-contaminated prosthetic joint arthroplasties, do not administer additional antimicrobial prophylaxis doses after the surgical incision is closed in the OR, even in the presence of a drain.
(Singhal, 2019)
PREVENTING CENTRAL LINE–ASSOCIATED BLOODSTREAM INFECTIONS
AHRQ (2018a) guidelines for prevention of CLABSIs include the following:
Catheter Insertion
- Use aseptic technique:
- Use appropriate hand hygiene using soap and water or a waterless hand sanitizer.
- Use face mask, cap, and sterile gloves.
- Wear a sterile gown with neck snaps and wrap-around ties properly secured.
- Instruct anyone assisting to wear the same barriers.
- Cover the patient entirely with a large sterile drape.
- Create a sterile working surface that acts as a barrier between the insertion site and any possible source of contamination.
- Prepare skin with antiseptic/detergent chlorhexidine 2% in 70% isopropyl alcohol.
- Apply a sterile dressing to the insertion site before the sterile barriers are removed.
- Transparent dressings are preferred to allow visualization of the site.
- Use chlorohexidine for skin preparation.
- Use full barrier precautions during central venous catheter insertion.
- Avoid using the femoral vein for catheter in adult patients.
CV Catheter Site Selection
- Use the subclavian site unless medically contraindicated (anatomic deformity, coagulopathy, renal disease that may require dialysis).
- If the internal jugular vein is chosen, use the right side to reduce risk of noninfectious complications since it has a larger diameter and a straighter path to the superior vena cava.
CV Catheter Selection
- Use a single-lumen central venous access device (CVAD) for patients requiring long-term access (more than 30 days) or a PICC or cuffed CVAD for patient requiring access for greater than 2 weeks.
Arterial Line Site Selection
- Radial artery is the preferred site.
- Dorsalis pedis is the alternative site.
- Femoral sites have higher infection rates, brachial/maxillary site are last resort.
Postinsertion Care
- Evaluate the need for CVAD daily.
- Remove catheter when not needed or change to a single-lumen CVAD when possible.
- Replace the dressing when it becomes damp, loosened, or soiled.
- Replace gauze dressing used on short-term central venous catheter (CVC) sites every 2 days.
PREVENTING PERIPHERAL IV CATHETER–RELATED BLOODSTREAM INFECTIONS
Guidelines for prevention of peripheral IV catheter–related bloodstream infections include the following:
Site Selection
- In adults, use an upper-extremity site for catheter insertion.
- In pediatric patients, use the upper or lower extremities or the scalp (in neonates or young infants).
Catheter Selection
- Avoid use of steel needles for administration of fluids and medications that might cause tissue necrosis if extravasation occurs.
- Use a midline catheter or peripherally inserted central catheter (PICC), instead of short peripheral catheter, when duration of IV therapy will exceed six days.
Catheter Insertion
- Perform hand hygiene before insertion.
- Prepare clean skin using a chlorhexidine-based solution. If contraindicated, tincture of iodine, an iodophor or 70% alcohol can be used. Allow to dry prior to placing catheter.
- Maintain aseptic technique for insertion of peripheral IV (PIV).
- Wear clean gloves, rather than sterile, for insertion of a peripheral intravenous catheter if the access site is not touched after application of skin antiseptics.
- Use maximal sterile barrier precautions (cap, mask, sterile gown, sterile gloves, and sterile full body drape) for insertion of PICCs.
- Use either sterile gauze or sterile, transparent, semipermeable dressing to cover site.
Catheter and Site Care
- Perform hand hygiene procedures before and after palpating catheter insertion sites as well as before and after, replacing, accessing, repairing, or dressing an intravascular catheter.
- Evaluate catheter insertion site daily both visually and by palpation through the dressing to discern tenderness and by inspection if a transparent dressing is in use. If local tenderness or other signs of possible infection occur, an opaque dressing should be removed and the site inspected visually.
- Replace catheter site dressing if it becomes damp, loosened, or visibly soiled.
- Do not use topical antibiotic ointment or creams on insertion sites, except for dialysis catheter.
- Remove peripheral venous catheter if patient develops signs of phlebitis, infection, or a malfunctioning catheter.
- Wear either clean or sterile gloves when changing the dressing on catheter sites.
- Replace dressing every 7 days for transparent dressing, except in pediatric patients in which risk for dislodging catheter may outweigh the benefit.
- Do not submerge catheter or catheter site in water; cover during showering.
- In both adult and pediatric patients, leave peripheral venous catheters in place until IV therapy is completed, unless a complication occurs.
- For catheters inserted under emergency conditions, insert a new catheter at a different site within 24 hours.
- Encourage patients to report any changes in their catheter site or any new discomfort to their provider.
Replacement of Administration Sets
- Replace administration sets, including secondary sets and add-on devices, no more frequently than at 96-hour intervals, unless clinically indicated.
- Replace tubing used to administer blood, blood products, or lipid emulsions within 24 hours of initiating the infusion.
(Jacob & Gaynes, 2020; CDC, 2017a)
PREVENTING CLOSTRIDIOIDES DIFFICILE INFECTIONS (CDIs)
Strategies for the prevention of Clostridioides (formerly known as Clostridium) infection include the following:
- Isolate and initiate Contact Precautions for suspected or confirmed CDI.
- Maintain Contact Precautions for at least 48 hours after diarrhea has resolved, or longer, up to the duration of hospitalization.
- Adhere to recommended hand hygiene practices.
- Use dedicated patient-care equipment (e.g., blood pressure cuffs, stethoscopes).
- Implement daily patient bathing or showering with soap and water.
- When transferring patients, notify receiving wards or facilities about the patient’s CDI status.
- Perform daily cleaning of CDI patient rooms using C. difficile sporicidal agent at least once a day, including toilets.
- Clean and disinfect all shared equipment prior to use with another patient (e.g., wheelchair).
- Perform terminal cleaning after CDI patient transfer/discharge using a C. difficile sporicidal agent.
- Clean additional areas that are contaminated during transient visits by patients with suspected or confirmed CDI (e.g., radiology, emergency rooms, physical therapy) with C. difficile sporicidal agent.
- Restrict use of antibiotics with the highest risk for CDI (e.g., fluroquinolones).
- Ensure that patients receive the shortest effective duration of antibiotic therapy.
- Limit use of nonantibiotic patient medications (e.g., proton pump inhibitors, H2-receptor blockers) that are hypothesized to increase risk for CDI.
- Consider additional disinfection of CDI patient room with no-touch technologies (e.g., UV light).
- Dedicate healthcare personnel to care only for patients with CDI only to minimize risk of transmission to others.
(CDC, 2017b)
PREVENTING MULTIDRUG-RESISTANT ORGANISM (MDRO) INFECTIONS
The CDC recommends the use of Contact Precautions in inpatient acute care settings for patients known to be colonized or infected with epidemiologically important MDROs, including methicillin-resistant Staphylococcus aureus (MRSA). However, there is debate as to the most beneficial way to manage patients with MDRO infections. Despite current guidelines, cluster-randomized trials have failed to show a benefit of initiating Contact Precautions over usual care for the prevention of MRSA or vancomycin-resistant enterococci (VRE) infections in hospitals.
Based on current evidence, however, the CDC continues to recommend the use of Contact Precautions for MRSA-colonized or -infected patients. The CDC will continue to evaluate the evidence on Contact Precautions as it becomes available.
In acute care hospitals, CDC recommendations state:
- Promote the judicious use of antimicrobial agents.
- Follow Standard Precautions during all patient encounters in all healthcare settings.
- Use a mask according to Standard Precautions when:
- Performing a splash-generating procedure
- Caring for patients with open tracheostomies
- In circumstances where there is evidence of transmission from heavily colonized sources, such as burn wounds
- Not recommended during routine care
- Implement Contact Precautions for all patients known to be colonized/infected with target MDROs.
In long-term care facilities:
- Consider the individual patient’s clinical situation and prevalence or incidence of MDROs in the facility when deciding whether to implement or modify Contact Precautions in addition to Standard Precautions for a patient infected or colonized with a target MDRO.
In ambulatory and home care settings:
- Follow Standard Precautions.
- Limit the amount of reusable patient care equipment that is brought into the home of patients infected or colonized with MDROs.
(CDC, 2020c)
PREVENTING VENTILATOR-ACQUIRED LUNG INFECTIONS
Strategies for the prevention of ventilator-associated pneumonias includes:
- Use of routine infection control practices and hand hygiene
- Prophylactic antibiotic administration
- Sedation interruption
- Keeping head of bed elevated 30 to 45 degrees
- Limitation of ventilation times
- Endotracheal suctioning
- Avoiding gastric overdistention
- Draining ventilator tube condensate
- Kinetic bed therapy
- Changing ventilator circuit if visibly soiled or mechanically malfunctioning
- Using sterile suctioning techniques and handling of respiratory equipment
- Performing oral care at least every 2 to 4 hours with an antiseptic swab to clean the oral cavity and teeth; brushing the teeth twice a day. Antiseptics to include:
- Chlorhexidine in different applications as oral rinse, gel, or foam
- Povidone-iodine 10%
Falls
Falls are the most common type of accidents in people 65 years of age and older, with over 30% of such individuals falling every year. In approximately one half of these cases, the falls are recurrent. These percentages increase to around 40% in individuals 85 years and older.
Approximately 10% of falls result in serious injuries, including fracture of the hip, other fractures, traumatic brain injury, or subdural hematoma. They are the major cause of hospitalization related to injury in those 65 years and older and are associated with increased mortality. The associated use of ambulance services, social care, and hospital care results in substantial financial costs.
Falls in institutional settings occur more frequently and are associated with greater morbidity than falls that occur in the community. Approximately 50% of individuals in the long-term care setting fall yearly (Appeadu & Bordoni, 2020; Kiel, 2020).
FALL RISKS
The Joint Commission identifies the most common contributing factors to falls with injury as follows:
- Inadequate assessment
- Communication failures
- Lack of adherence to protocols and safety practices
- Inadequate staff orientation or supervision
- Inadequate staffing levels or skill mix
- Deficiencies in the physical environment
- Lack of leadership
(TJC, 2021b)
Falls risk can be categorized as either intrinsic or extrinsic. Intrinsic factors include issues that are unique to the individual and concern medical, psychological, and physical issues. Extrinsic factors generally can be changed and address environmental risks that patients encounter.
Intrinsic Risk Factors
- Advanced age
- Previous falls
- Gender (women fall more often than men)
- Race (Whites fall most often)
- Taking more than four medications
- Lower extremity weakness
- Impairment in gait and mobility
- Immobility/deconditioning
- Post-fall anxiety syndrome (fear of falling following a recent fall)
- Inner ear disorders
- Cardiovascular or cerebrovascular disorders
- Cognitive disorders
- Poor nutrition
- Poor vision
- Alcohol use
- Postural hypotension
- Chronic conditions such as arthritis, stroke, incontinence, diabetes, Parkinson’s disease
- Foot problems leading to balance issues
(Appeadu & Bordoni, 2020)
Extrinsic Risk Factors (account for 30% to 50% of falls in the older population)
- Lack of stair handrails
- Lack of bathroom grab bars
- Insecure toilet seat or handrail
- Low toilet seat
- Dim lighting or glare
- Obstacles and tripping hazards
- Slippery or uneven surfaces, raised thresholds, missing floor tiles
- Unstable or lightweight furniture
- Hard-to-reach personal items
- Improper use of assistive devices
- Ill-fitting or inappropriate footwear
- Hard-to-manage clothing
- Use of restraints
- Wheelchair issues (e.g., missing parts, incorrect fit, inadequate seating)
(AHRQ, 2017b)
Older patients are not the only population at risk. Any patient who has had excessive blood loss may experience postural hypotension, increasing the risk of falling. Maternity patients or other patients who have epidural anesthesia are at risk for falls due to decreased lower-body sensation (AHRQ, 2017b).
PREVENTING FALLS
Preventing falls involves assessing patients for risk for falls, developing a personalized plan of care, and utilizing consistent preventive interventions. Fall prevention interventions are to be considered in both hospitalized and ambulatory settings.
Hospitalized Patients
Risk factors for falls and injury in hospitalized patients include:
- Age or frailty
- Osteoporosis or a recent fracture
- Bleeding disorders/taking anticoagulants
- Recent surgery
A fall risk assessment should be done on admission, and reassessment should be done whenever there is a change in a patient’s condition or when a patient is being transferred to another unit. A reliable, standardized, and validated assessment scale should be used that includes a history of falls, mobility problems, use of assistive devices, medications, and mental status.
While some institutions have created their own assessment tools, tools that have been extensively studied and recommended include:
- Morse Fall Scale
- STRATIFY Scale
- Schmid Fall Risk Assessment Tool
After assessment of fall risk, collaboration with the patient and family takes place in order to develop a personalized plan that addresses each identified risk factor. Tailored prevention interventions may include:
- For gait instability/lower-limb weakness:
- Nonskid footwear
- Assistive devices
- Physical therapy evaluation/treatment
- Assistance getting out of bed and with ambulation
- Avoiding bedrest
- For urinary incontinence, frequency, and/or need for toileting:
- Hourly rounding
- Toileting schedule
- Incontinence briefs
- For agitation, confusion, or impaired judgment:
- Frequent rounding/surveillance plan
- Activity schedule
- Continuous virtual monitoring
- Bed/chair alarms
- Floor mats to reduce trauma from bed-related falls
- Assess for alcohol or drug withdrawal, place on appropriate protocol
- Rule out delirium
- Due to medications:
- Consulting the pharmacist
- Assessing for and treating orthostatic hypotension
- Assessing for medication side effects
- Avoiding hypnotics
(Dykes et al., 2018)
Community-Dwelling Patients
The Centers for Disease Control and Prevention’s STEADI (Stop Elderly Accidents, Deaths, and Injuries) initiative is a coordinated approach for the implementation of practice guidelines for fall prevention in community-dwelling adults. The STEADI initiative is a coordinated approach to implementing clinical practice guidelines for fall prevention that consists of the three core elements of screen, assess, and intervene. The STEADI Algorithm for Fall Risk Screening, Assessment, and Intervention outlines how to implement these three elements, as follows:
- Screen for fall risk annually, or any time the patient presents with an acute fall:
- For patients found not at risk, prevent future risk by recommending effective prevention strategies:
- Education patient about fall prevention.
- Assess vitamin D intake and recommend supplement if deficient.
- Refer to community exercise or fall prevention program.
- Reassess yearly or any time the patient presents with an acute fall.
- For patients found not at risk, prevent future risk by recommending effective prevention strategies:
- Assess those who are found to be at risk:
- Assess the patient’s modifiable risk factors and fall history and evaluate gait, strength, and balance. Common assessments include:
- Timed Up and Go (TUG)
- 30-second Chair Stand
- 4-stage Balance Test
- Identify medications that increase fall risk (e.g., using Beers criteria) (see “Resources” at the end of the course).
- Ask about potential home hazards.
- Measure orthostatic blood pressure.
- Assess visual acuity (Snellen eye test).
- Assess feet and footwear.
- Assess vitamin D intake.
- Identify comorbidities (e.g., depression, osteoporosis).
- Assess the patient’s modifiable risk factors and fall history and evaluate gait, strength, and balance. Common assessments include:
- Intervene to reduce identified risk factors:
- Discuss patient and provider health goals and develop an individualized patient care plan.
- Poor gait, strength, and balance observed:
- Refer for physical therapy evaluation.
- Refer to evidence-based exercise or fall prevent program. - Medication(s) likely to increase fall risk:
- Optimize medications by stopping, switching or reducing dosages of medication. - Home hazards as described:
- Refer to occupational therapist to evaluate home safety.
- Poor gait, strength, and balance observed:
- Orthostatic hypotension observed:
- Stop, switch, or reduce dose of medications that increase fall risk.
- Educate about importance of exercises (e.g., foot pumps).
- Establish appropriate blood pressure goal.
- Encourage adequate hydration.
- Consider compression stockings.
- Visual impairment observed:
- Refer to ophthalmologist/optometrist.
- Stop, switch, or reduce dosage of medication affecting vision (e.g., anticholinergics).
- Consider benefits of cataract surgery.
- Provide education on depth perception and single-vision multifocal lenses.
- Feet/footwear issues identified:
- Provide education on shoe fit, traction, insoles, and heel height.
- Refer to a podiatrist.
- Vitamin D deficiency observed or likely:
- Recommend daily vitamin D supplement.
- Comorbidities documented:
- Optimize treatment of conditions identified.
- Be mindful of medications that increase fall risk.
- Follow-up with the patient in 30 to 90 days to discuss ways to improve patient receptiveness to care plan and to address barrier(s).
- Discuss patient and provider health goals and develop an individualized patient care plan.
MORSE FALL SCALE (MFS)
The MFS is used widely in both hospital and long-term care inpatient settings. The MFS requires systematic, reliable assessment of a patient’s fall risk factors upon admission, after a fall, upon change in status, and at discharge or transfer to a new setting. MFS subscales include assessment of:
| Risk Factor | Score |
|---|---|
| History of falling, immediate or within 3 months |
No = 0 |
| Yes = 25 | |
| Secondary diagnosis | No = 0 |
| Yes = 15 | |
| Ambulatory aid | None, bed rest, wheelchair, nurse = 0 |
| Crutches, cane, walker = 15 | |
| Furniture = 30 | |
| IV/heparin lock | No = 0 |
| Yes = 20 | |
| Gait/transferring | Normal, bed rest, immobile = 0 |
| Weak = 10 | |
| Impaired = 20 | |
| Mental status | Oriented to own ability = 0 |
| Forgets limitations = 15 |
| MFS Score | Risk Level | Action |
|---|---|---|
| 0–24 | None | Basic nursing care |
| 25–50 | Low | Standard fall prevention interventions |
| 51+ | High | High-risk fall prevention interventions |
(AHRQ, 2018b)
ROLE OF PHYSICAL THERAPY IN FALL PREVENTION
For patients who are found to be at fall risk, a physical therapist will perform a thorough evaluation, including:
- Review of medical history
- Review of medications
- Simple vision test
- Balance, strength, range of motion, and walking ability
- Home safety assessment
- Simple test of cognitive abilities
- Assess orthostatic blood pressures
- Assess feet and footwear
Based upon the findings, the physical therapist designs a treatment plan tailored to the patient’s needs, which may include:
- Balance training
- Prescribed exercise program that includes walking and/or gait training
- Dual-task training program
- Strength training
- Endurance training
- Pain management
- Education on nutrition, sleep, choosing appropriate footwear
- Fear management
- Referral to community programs
- Home safety guidance
(APTA, 2018)
ROLE OF OCCUPATIONAL THERAPY IN FALL PREVENTION
Occupational therapists consider how the person functions in their day-to-day environment. They assess for hazards and patient limitations that contribute to falls and offer safety education to patients and/or caregivers during activities of daily living. Occupational therapists can also earn specialty certification in fall prevention.
Fall risk factors addressed by occupational therapy include:
- Lower-extremity weakness
- Impaired balance
- Cognitive impairment
- Urinary incontinence
- Sensory impairment
- Fear of falling
- Side effects of medication
- Throw rugs and loose carpeting
- Lighting and glare
- Pets
- Clutter
- Uneven sidewalks and thresholds
- Unstable or nonexistent handrails
(AOTA, 2021)
Health Information Technology (IT) Problems
Healthcare facilities across the United States have made great efforts in moving from paper to electronic systems and processes since the Health Information Technology for Economic and Clinical Health (HITECH) Act was passed in 2009. But while health IT systems have great potential to improve patient safety and help ensure high-quality healthcare, they can also present unintended risks. As these systems become more and more integrated into the delivery of healthcare and the monitoring of safety and quality, how providers interact with these systems as users has become increasingly significant.
Health IT that is poorly designed or poorly applied may contribute to miscommunication, delay, confusion, or distraction. Decision support tools that are not usable may be ignored or, worse, interfere with the delivery of high-quality patient care. In addition, adverse events with the potential for causing significant harm, such as delayed diagnosis, medication errors, and incorrect treatment decisions, have been associated with the use of health IT.
Training is essential to ensure that all users understand how to operate the technology in a safe manner and take advantage of its time-saving features. Feedback from end users over time can help drive improvements in systems, processes, and training resources (Health IT.gov, 2019; AHA, 2018).