TREATING HEART FAILURE
There are several levels of treatment parameters for heart failure. While there is no cure, the treatment goals are:
- Symptom management
- Prevention of exacerbation
- Prevention of advancement to worse functional classifications
Collaborative management refers to the combined efforts of the various healthcare team members who combine therapies and areas of expertise to manage symptoms and prevent exacerbations. A well-coordinated, collaborative approach can also prevent advancing to a worsening level of function.
Medications may be used to improve cardiac output, reduce cardiac workload, improve symptoms, reduce mortality, and reduce the occurrences of readmission to the hospital.
Surgical interventions such as heart valve replacement or repair, internal cardiac defibrillators, transtelephonic electrocardiographic transmission devices, coronary artery bypass grafts, or heart transplants are used to treat and repair some of the underlying causes of HF, reduce dysrhythmia, improve organ perfusion, and reduce mortality.
Cardiac assist devices serve to improve organ perfusion.
Supportive devices treat symptoms and reduce the occurrence and length of hospitalizations. Human embryonic stem cell therapy can reduce cardiac tissue necrosis. Cardiac monitoring devices continually collect and analyze cardiac-related data and can alert the clinician to the presence of important physiological events, often before symptoms occur.
Nutritional therapy helps to promote weight reduction and prevent edema.
Collaborative Management
Patients with heart failure receive treatment from a wide spectrum of healthcare professionals. Acute and chronic phases of the disease therapy, including cardiac rehabilitation, require specialists to deal with the assorted aspects of needed care. The individual disciplines may include physicians, different levels of nurses (RN, advanced practice, vocational or practical nurse), physical therapists, occupational therapists, pharmacists, respiratory therapists, nutritionists or registered dietitians, mental health professionals, and social workers.
The use of a coordinated, multidisciplinary heart failure team results in improved outcomes for HF patients both when hospitalized and as outpatients. The introduction of such teams to provide expertise in the initiation and management of therapy decreases inpatient and one-year mortality rates, hospital length of stay, and the number of hospital readmissions for HF (Boa Sorte Silva et al., 2020).
CASE
Miguel is in the hospital for the fourth time for symptoms of heart failure. He is 79 years old, moderately obese, has type 2 diabetes, and has a stage 3 pressure injury (ulcer) on his heel. He is unable to walk because of pain from the ulceration. He and his family are native Spanish speakers.
Miguel’s physician has determined that the patient is stable and ready for discharge but also recognizes that there are some barriers to his being well cared for at home, where he lives with his daughter. Miguel’s daughter has not yet had the opportunity to practice dressing changes on Miguel’s heel ulcer due to language barriers with the inpatient nursing staff. Miguel’s heel ulcer has affected the stability of his gait as well as his standing balance for functional tasks, which poses a safety risk in the home setting. Miguel’s daughter has also told the nurse that the food choices suggested by the inpatient dietitian did not make any mention of culturally specific foods that her father most enjoys.
Miguel’s physician determines that Miguel and his family need instruction in diet, wound care, and mobility, particularly ambulation and strength training. On the physician’s orders, Miguel is seen by a certified wound care/ostomy nurse, the dietitian, a physical therapist, an occupational therapist, and a discharge planner before his discharge.
The wound care nurse makes a thorough examination of the heel ulceration. She orders a MediHoney paste, applies it to the wound, and dresses it. She is fluent in Spanish and explains to Miguel’s daughter how to treat the wound when he goes home. She gives his daughter samples of the MediHoney and some dressing material and explains that she can obtain more in any pharmacy or grocery store with a pharmacy section.
The dietitian uses the services of a certified medical translator to instruct the daughter in preparing a calorie- and carbohydrate-limited ADA diet for Miguel. She asks for suggestions from the daughter about Miguel’s favorite foods and incorporates some of the daughter’s Mexican specialties into the diet plan, with instructions on substitutions for traditional high-carbohydrate foods like tortillas, beans, and rice.
The physical therapist evaluates Miguel and recommends the use of a rolling walker with partial weight-bearing for all ambulation while his foot wound heals. He provides gait training to instruct Miguel in partial weight-bearing in order to minimize pressure on his heel wound. The physical therapist also educates both Miguel and his daughter regarding the importance of regular ambulation to improve circulation and prevent edema.
The occupational therapist evaluates Miguel and observes his execution of ADLs (activities of daily living) such as hygiene, grooming, eating, and dressing. She suggests that the patient may benefit from the use of a covered cup for liquid intake and large utensils to facilitate eating since his advanced age and diabetes have caused visual impairment. The occupational therapist also suggests the use of a weekly medication holder to help the daughter organize Miguel’s daily medications to prevent omissions or errors.
The discharge planner also uses the services of the medical interpreter to arrange for admission to a rehabilitation facility to help Miguel with functional mobility and to prepare him to eventually be discharged home.
Pharmacologic Interventions
MEDICATIONS TO REDUCE CARDIAC WORKLOAD
In HF there are many factors that can increase the workload of the heart. Excess circulating volume forces the heart to work harder to pump an increase of fluid throughout the body. Stress may produce a rapid heart rate, causing the heart to work overtime. Hypertension reflects vascular resistance against which the heart must pump harder to produce cardiac output. Antihypertensives exert individual chemical properties to control physical response to sympathetic nervous system stimulation, vasoconstriction, or fluid overload secondary to sodium and fluid retention.
Aldosterone (mineralocorticoid receptor) antagonists are used in HF to promote diuresis as a means of lowering blood pressure to reduce myocardial tissue damage, cardiac myopathy, and fibrosis. These have shown to reduce mortality and hospital readmission in HF patients. Examples include spironolactone and eplerenone (Kruik-Kollöffel et al., 2020).
Angiotensin converting enzymes (ACE) inhibitors reduce vascular resistance by interfering with the renin-angiotensin-aldosterone system (RAAS) to reduce the conversion of angiotensin I to angiotensin II. This decreases aldosterone excretion and sodium retention to reduce blood pressure and improve blood flow. Angiotensin II is a powerful vasoconstrictor short-term and over the long run affects the blood vessels’ tissue growth that results in remodeling of the vessel walls and causes hypertension. Examples include enalapril (Vasotec), lisinopril (Zestril), and captopril (Capoten). Asian Americans have a high (up to 50%) risk for ACE inhibitor–induced cough as a side effect (Harding et al., 2020; Sole et al., 2020).
Angiotensin receptor blockers (ARBs) similarly affect the RAAS to reduce the pressure in the heart and may be prescribed for those who cannot take ACE inhibitors due to chronic cough or angioedema. These drugs include losartan (Cozaar) and valsartan (Diovan) (Sole et al., 2020).
Angiotensin receptor-neprilysin inhibitors (ARNIs) are a newer classification of drugs for the treatment of HF. The FDA has approved sacubriril/valsartan (Entresto) as the first drug in this category to be used with NYHA classes II, III, or IV. This drug has two main effects: the first, from the valsartan, is a similar effect to ACE inhibitors and ARBs (see above); the second, from the sacubritil, strengthens a hormone regulated by the heart muscle itself, which releases BNP. BNP causes vasodilatation and increases sodium excretion by the kidneys and an increased diuresis. These result in a significant reduction in hospitalization and mortality. Known side effects are hypotension, dizziness, syncope, hypovolemia, hyponatremia, cough, and renal insufficiency (Docherty et al., 2020).
Vasodilators increase the internal diameter of the blood vessels to promote better blood flow and reduce blood pressure.
Nitrates cause vasodilation and increased venous capacity by relaxing the smooth muscle of blood vessel walls. When HF accompanies myocardial ischemia secondary to CAD, nitrates are particularly beneficial, causing coronary artery dilation and improving blood flow to the myocardium to relieve or prevent chest pain. Examples of nitrates are isosorbide dinitrate (Isordil) and sublingual nitroglycerin. The most common side effects of nitrates are vasodilation of peripheral arteries, resulting in reduced venous resistance and lowered blood pressure, and headache. African Americans are the only ethnic group for whom the combination drug isosorbide dinitrate/hydralazine (BiDil) is approved as treatment for HFrEF (Harding et al., 2020; Sole et al., 2020).
Loop diuretics promote renal excretion of fluids and sodium chloride to decrease the circulating intravascular volume, which reduces blood pressure. This action takes place in the ascending loop of Henle in the renal tubules. Excretion of excess fluid reduces cardiac workload and oxygen consumption. Examples of loop diuretics are furosemide (Lasix), bumetanide (Bumex), and torsemide (Demadex).
Vasopressin-2 receptor antagonist tolvaptan (Jynarque, Samsca) in renal failure patients with HF, when added to conventional therapy, has shown improved dyspnea symptoms, lower doses of loop diuretics needed, and increased urine output than for those patients treated with loop diuretics alone. This has proven effective for renal dysfunction patients, since they are usually refractory to loop diuretics, requiring higher doses that are themselves the cause of increased renal dysfunction. Tolvaptan is a selective vasopressin-2 receptor antagonist that acts on the distal portion of the nephron and inhibits the kidney’s ability to reabsorb water (Sole et al., 2020).
MEDICATIONS TO IMPROVE CARDIAC OUTPUT
Positive inotropic agents increase the force of cardiac contractions (inotropic effect) to improve cardiac output. Some reduce the heart rate (chronotropic effect), allowing for more complete ventricular filling to increase stroke volume. Catecholamines are positive inotropic agents given to HF patients with severe disease. They are powerful vasoconstrictors, acting to support failing blood pressure.
- Dopamine and norepinephrine are endogenous catecholamines used in severe or end-stage HF to increase contractility, but which also cause increased cardiac workload and dysrhythmias. They may be used to support a failing heart that awaits transplantation.
- Dobutamine is a synthetic catecholamine with similar actions but that does not increase systemic vascular resistance.
- Milrinone is a widely used inotrope that increases myocardial contractility by inhibiting phosphodiesterase and thereby allowing an influx of calcium into the myocardial cells. Milrinone increases cardiac output by reducing BP through vasodilation. It may not be used in patients with renal disease, HF caused by ischemia, or hypotension.
Heart failure patients who take digitalis preparations experience a reduction in symptoms, expanded exercise tolerance, and improved quality of life. They do not experience decreased mortality rates, however. Digitalis preparations present a significant risk for toxicity, particularly in the presence of electrolyte imbalance. Hyper- or hypokalemia, hypercalcemia, and hypomagnesemia may cause potentially fatal dysrhythmias in patients taking digitalis. The most common medication in this category is digoxin (Lanoxin) (Sole et al., 2020).
GUIDELINE-DIRECTED MEDICAL THERAPY (GDMT)
In a recent study, GDMT proved to reduce the mortality for patients with HFrEF as well as patients with coronary artery disease in need of surgery. Giving the patients the combination of one or more antiplatelet drugs, a statin to reduce serum cholesterol and triglycerides, a beta-blocker to reduce cardiac workload, and an angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB) reduced mortality rates compared to those with the same diagnoses on other drug regimens (Wolfe et al., 2020).
MISCELLANEOUS MEDICATIONS
Three specific beta-adrenergic blocking agents (beta blockers) have been shown to reduce mortality in patients with HF with reduced ejection fraction (HFrEF): metoprolol succinate (Toprol XL), bisoprolol (Zebeta), and carvedilol (Coreg). These may also have a dose-related effect, increasing the EF and therefore increasing cardiac output. Beta blockers are started at low dose, as they may reduce cardiac contractility (Harding et al., 2020).
The heart failure agent (hyperpolarization-activated cyclic nucleotide gated channel blockers) ivabradine (Corlanor) is a pharmaceutical treatment specifically for heart failure that is designed to prevent patient readmission to the hospital. It may be used in patients with a stable but symptomatic reduced ejection fraction of <35%, sinus rhythm, and a HR >70. It acts by reducing the heart rate, thus preventing the disease process from progressing. Side effects are phosphenes (visual disturbances) and dysrhythmias, the most common of which is bradycardia. Ivabradine is given to HF patients who are already taking the maximum dose or are unable to take beta-blocking agents (Amgen, 2019).
B-type natriuretic peptide (BNP) treatments given subcutaneously show promise due to the capacity to improve left ventricular function and urine output. The prototype is the recombinant human BNP nesiritide (Natrecor). Initial studies of BNP treatment to prevent recurrence of heart failure symptoms and hospital readmissions showed that the subcutaneous route of administration did not cause hypotension as had previous studies with intravenous medications. Further studies are needed to prove the efficacy of subcutaneous BNP treatments (Sole et al., 2020).
PHARMACOLOGIC AGENTS HELD OR USED WITH CAUTION IN HEART FAILURE
Antidysrhythmic agents are generally withheld in HF, except amiodarone given for ventricular dysrhythmias. Other antidysrhythmic agents may exacerbate the symptoms of heart failure.
NSAIDs, ACE inhibitors, and diuretics when given in combination may cause renal damage as evidenced by a reduced glomerular filtration rate (GFR). NSAIDs reduce the synthesis of vasodilatory prostaglandins and may inhibit the systemic antihypertensive effect of ACE inhibitors. As ACE inhibitors and diuretics are often used in the treatment of HF, it is recommended that other mid-level range pain relievers be used instead, such as acetaminophen (Tylenol). Anti-inflammatory medications such as ibuprofen may cause toxicity when used in conjunction with calcium channel blockers, as are commonly used in HF. Anti-inflammatory medications and immunomodular agents such as infliximab often cause fluid retention, which can exacerbate edema in patients with HF.
Thiazolidinediones, also known as glitazones, such as pioglitazone and rosiglitazone are insulin activators that may increase sodium retention and aggravate HF symptoms in patients with diabetes with comorbidities for both diseases. These medications may cause fluid retention, edema, and weight gain in class III and IV HF. This may necessitate readmission to the hospital for HF because of the exacerbation of symptoms (McCuistion et al., 2019).
Beta-adrenergic blocking agents in acute HF are used judiciously because of the possibility of reducing cardiac contractility and therefore the strength of the cardiac chambers pumping blood to the rest of the body. When beta blockers are used in HF, they are started in the lowest possible doses to achieve therapeutic effect, and the doses are then titrated up slowly if more drug is needed (Harding et al., 2020). Both the patient’s providers and nurses monitor vital signs closely and observe for signs of reduced cardiac contractility such as blood pressure variations, tachycardia, auscultation of an S3 heart sound, or pulsus alternans (a pulse that alternates strong and weak on palpation).
METFORMIN RESTRICTIONS DROPPED
A meta-regression analysis of changes in the U.S. Food and Drug Administration boxed warning regarding the use of Metformin suggests that previous prohibitions with giving metformin to HF patients no longer hold. Metformin use in chronic diseases such as kidney disease, liver disease, and heart failure are no longer believed to increase mortality, so the restrictions have been dropped. In fact, evidence shows that the use of metformin in HF with preserved ejection faction (HFpEF) reduces mortality and hospitalization rates. The drug is especially effective with patients with an ejection fraction >50% (Halabi et al., 2020).
Surgical Interventions
Surgeries can be performed to repair conditions that contribute to severe HF, particularly when nonsurgical treatments alone are ineffective. When the underlying cause of heart failure is corrected, such as by a cardiac valve replacement, the condition itself may improve, depending on the severity of the disease. Hypertrophy of the myocardium, for example, particularly the left ventricle, can only be repaired by a cardiac transplant. (The degree of damage to the myocardium, however, may make the patient a poor candidate for transplantation.)
VALVE REPLACEMENT / VALVE REPAIR
Cardiac valve malfunction can be caused by either stenosis that does not allow the blood to flow through to the next chamber or prolapse that prevents the valve from closing completely (atresia), causing regurgitation. Depending on the volume of blood allowed to remain in the cardiac chambers, this regurgitation may cause the affected chamber to have to pump harder to empty, resulting in HF. Of concern is left ventricular dysfunction, as cardiac output is then compromised, causing HF.
The most common valve to be repaired via a surgical procedure is the mitral valve, which prevents regurgitation from the left ventricle into the left atrium. The most common type of valve to be replaced via a surgical procedure is the aortic valve, which prevents regurgitation from the aorta into the left ventricle. The pulmonic and tricuspid valves rarely undergo replacement or repair.
Valve Repair
Valve repair surgery is a closed-heart procedure requiring the patient’s heart to be monitored continuously by a nurse or monitor technician specially trained to recognize dysrhythmias on the cardiac monitor. This patient will be cared for by a cardiologist as well. The placement of clips to prevent the backflow of blood is the most common method of repair of the mitral valve. The average length of stay in the hospital following valve surgery is two to seven days depending on the degree of invasiveness inherent in the procedure.
Types of surgical valve repair include:
- Patching holes or tears with tissue to provide more support at the base of the valve
- Removing or reshaping tissue so that the valve can close more effectively
- Separating valve flaps that are fused due to a congenital defect
Valve Replacement
The traditional method for valve replacement requires open-heart surgery, necessitating the patient be transferred to the intensive care unit. Care is provided by critical care nurses specially trained to recover such a patient as well as a respiratory therapist if the patient requires a ventilator. A thoracic surgeon performs the surgery, and the patient is also cared for in the postoperative phase by a cardiologist.
The surgeon may use a biological valve from either pig, cow, or human heart tissue, which will last approximately 15 years and require anticoagulation. An artificial valve may also be used; it will last longer but requires that the patient take anticoagulants for life.
Transcatheter aortic valve replacement (TAVR) is an interventional approach. In patients who are not good candidates for traditional valve replacement surgery, a TAVR may be tried. The approach is via an inflatable balloon catheter via the femoral artery or transapically through the ribs. The balloon places the artificial valve within the existing valve. When the balloon is inflated, the artificial valve expands. The new valve then displaces the old, damaged valve and begins to function in its place (NHLBI, 2021a).
IMPLANTATION OF A CARDIAC DEFIBRILLATOR
Ventricular fibrillation, a life-threatening dysrhythmia, occurs at an alarming rate in patients with HF. This is particularly true of those with coronary heart disease as a comorbidity. The ability to have an internal device, an internal cardiac defibrillator (ICD), sense and correct this dysrhythmia has the potential capability to save lives. The device is a small computer implanted in the chest or abdomen and connected to wires inserted into one to three cardiac chambers. When the computer reads a sustained ventricular rhythm, it administers a shock to attempt to restore a more normal heart rhythm (NHLBI, 2021b). Its battery is replaced approximately every 20 years.
A recent study found a 13% increase in life expectancy in HF patients who received an ICD compared to those with similar levels of HF without an ICD (NHLBI, 2021c).
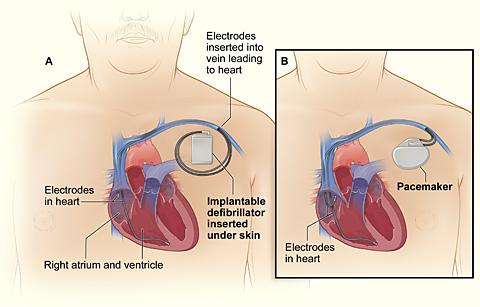
Comparison of an ICD and a pacemaker.
Figure A (left) shows the location and general size of an ICD in the upper chest; the wires with electrodes on the ends are inserted into the heart through a vein in the upper chest.
Figure B (right) shows the location and general size of a pacemaker in the upper chest; the wires with electrodes on the ends are inserted into the heart through a vein in the upper chest.
(Source: NHLBI, 2021b.)
CORONARY ARTERY BYPASS GRAFT / PERCUTANEOUS CORONARY INTERVENTION
One of the most common comorbidities with HF is coronary artery disease. The blocked coronary arteries of CAD inhibit the flow of blood, and therefore the oxygen bound to the hemoglobin molecule, to the myocardium, resulting in cardiac ischemia. If this disease progresses, an MI may occur. The infarcted area of the cardiac muscle then becomes akinetic, affecting the ability of the heart muscle to pump with its former strength. This causes the chambers of the heart to diminish in their ability to pump blood and empty with each contraction. If the area of the myocardium affected is the left ventricle, significant changes in the heart’s capacity for pumping blood to the rest of the body may be severely compromised, eventually resulting in HF.
CAD leads to angina pectoris and MI, the two components of acute coronary syndrome (ACS). As stated above, the occurrence of an MI can lead to heart failure by causing left ventricular dysfunction. Heart failure and left ventricular dysfunction are predictors of high mortality risk in patients with ACS. Earlier intervention with revascularization surgery—by grafting the patient’s own veins or arteries to bypass the obstructed coronary arteries—may prevent an MI and the subsequent HF. In a percutaneous coronary intervention (PCI) procedure, the coronary arteries are cleared by a percutaneous transluminal coronary angioplasty (PTCA) and held open by the placement of a stent. This is performed in less severe CAD or for patients who may be poor surgical candidates (Sole et al., 2020).
The patient is cared for in the ICU immediately postoperatively until the patient is extubated from the mechanical ventilator, temporary pacemaker wires have been removed, and the patient is hemodynamically stable. The patient will remain on a cardiac monitor throughout the entire hospital stay and will start on cardiac rehabilitation before being discharged.
HEART TRANSPLANTATION
Cardiac transplantation can be a life-saving measure and is most commonly performed in the case of end-stage HF that is refractory to medical treatment. In the case of HF caused by akinetic heart muscle or exacerbated by myocardial hypertrophy, nothing else will significantly alleviate symptoms, prevent progression of the disease, or prolong life.
This is a highly specialized surgery performed at only a handful of medical centers. The personnel involved in all aspects of this procedure are rigorously trained and are usually all critical care experts. In the United States, the list of patients awaiting cardiac transplantation is approximately 3,000, with approximately 2,000 hearts available per year.
Devices such as the A5000 Circulatory Support System and the BVS 5000 Biventricular Support System are FDA-approved as bridge-to-transplant (BTT) systems to support a failing heart until a matching donor heart is available. These are used as life-saving measures in the case where the patient would otherwise die but has a chance of recovery with a transplant. These patients are critically ill and monitored in an ICU while awaiting transplantation. (See also “Cardiac Assist Devices” below.)
The one-year post transplantation survival rate is 85% to 90%, and the three-year survival rate is 75%. The most common postoperative complications are rejection, infection, sudden cardiac death, malignancy, and cardiac vasculopathy (accelerated CAD) (Harding et al., 2020).
NURSE’S ROLE IN SURGERY
- Ensure diagnostic test results (e.g., laboratory tests, ECG, and X-rays) are on the chart that accompanies the patient to surgery or in the electronic medical record
- Obtain the patient’s signature for informed consent for surgery after the surgeon and anesthesiologist or nurse anesthetist have explained the procedures and possible negative outcomes to the patient (surgeon or anesthesiologist may also obtain the signed consent)
- Fill out a preoperative checklist, including a recent set of vital signs, how long the patient has been taking nothing by mouth (NPO), brief medical and surgical history, allergies, disposition of any belongings, surgical procedure to be performed, any medications taken that day, time of last voiding, and that the consent is signed
- Inform the family or any visitors where to wait until the surgery is completed (surgical waiting room) and let them know that someone will be in touch with them throughout the surgery
- For a patient with heart failure who is on vital cardiac or diabetes medications that should not be held due to the NPO status, administer with one sip of water per physician’s order
- For the operating room circulating nurse, just before the surgical procedure, conduct a “time out” with the surgeon, anesthesiologist, surgical technician, and anyone else involved in the surgery to identify and verify the patient and the procedure to be done
(Harding et al., 2020)
CASE
Alison is a certified respiratory therapist with 27 years of experience. She has worked the past 14 years at City Medical Center as the lead RT on the nightshift in the ICU. City Medical Center is an organ transplantation center, and Alison has seen dozens of heart, lung, and a few heart/lung transplants.
Tonight the ICU nurses are recovering a 68-year-old heart transplant patient with end-stage heart failure. He will be transferred out of the ICU when he becomes extubated, has his temporary pacemaker wires withdrawn, and is hemodynamically stable. The postoperative recovery is complicated by the fact that the patient has a comorbidity of moderately severe COPD and is having difficulty being weaned off the mechanical ventilator.
Working as a team, the ICU nurse, the pulmonologist on call, the anesthesiologist still in the hospital after surgery, and Alison work to get the patient extubated. The nurse gradually withdraws pain medication and sedation to allow the patient to become more alert and participate in his care. When he is sufficiently awake to breathe on his own, the patient becomes anxious, causing his heart rate and blood pressure to increase to abnormal levels. The anesthesiologist gives the order by phone for Alison and the ICU nurse to extubate the patient when he is responsive and the tidal volumes measured by Alison are normal. As the ICU nurse prepares to suction the patient, Alison deflates the pilot balloon on the endotracheal tube and slowly withdraws the tube from the patient’s throat. Together they complete a successful extubation.
Cardiac Assist Devices
Cardiac assist devices
INTRAAORTIC BALLOON PUMP (IABP)
This device reduces the systolic blood pressure to reduce afterload and thus decrease the cardiac workload and enhance the aortic diastolic pressure to improve coronary arterial blood flow. For HF patients, it works primarily as a bridge-to-transplant while they are hospitalized and waiting for an appropriate organ to be available.
A balloon is percutaneously inserted through the femoral artery into the thoracic aorta. The pump provides counterpulsation (opposite to the ventricular contractions) that causes the balloon to deflate and inflate, causing a rise in diastolic arterial pressure that improves coronary blood flow to the myocardium.
Contraindications include irreversible brain damage, major coagulopathy such as disseminated intravascular coagulation (DIC), terminal illnesses, abdominal or thoracic aortic aneurysms, moderate to severe aortic insufficiency, or generalized peripheral vascular disease.
Complications include dislodged plaque, aortic dissection, thromboembolism, compromised peripheral circulation, thrombocytopenia, blocked arteries, infection, and improper timing of inflation.
LEFT VENTRICULAR ASSIST DEVICE (LVAD)
An LVAD provides long- or short-term support for a heart that is failing. It is more compact than an IABP and allows the patient to be more mobile. The device may be inserted internally, such as in the peritoneum, or externally in a blood vessel. LVADs move blood from the left side of the heart to the device and then to the aorta to temporarily support circulation. They may be used in patients with HF for failure to wean from a cardiovascular bypass pump after open-heart surgery, after an MI, or as a bridge-to-transplant.
When the patient with heart failure is discharged from the hospital with such a device in place, daily function and the need to protect the device will affect the patient’s ADLs. An occupational therapist will assist the patient to manage the physical manipulation skills required to function with an LVAD. These may include placing the device in a fanny pack for ambulation or a waterproof case for showering, changing the batteries, and using fine motor control to manipulate the settings. By helping patients and their families learn adaptive skills to accommodate an LVAD, occupational therapists help HF patients maintain independence and return to work, school, and travel more safely (Abramson et al., n.d.).
Contraindications for an LVAD include small body surface area, renal or liver failure not following a cardiac event such as an MI, and comorbidities that limit life expectancy to less than three years. Complications are the same as for an IABP but are less likely to occur.

Left ventricular assist device. (Source: Singhvi & Trachtenberg, 2019.)
CASE
Jesse is an occupational therapist in a regional medical center. Jesse’s patient, Julia, a 74-year-old female, will be discharged with a newly implanted left ventricular assist device (LVAD). Julia is weak and lacks fine motor control in her fingers. Although the external controller on the device weighs only about five pounds, she complains that the fanny pack that houses the controller when she’s ambulatory “feels like a ton of bricks.”
Jesse works with Julia on improving her fine motor control to adjust the dials on the controller and teaches her how to change the battery on the device. Jesse also determines that the patient wasn’t wearing the pack correctly and helps her make the appropriate adjustments for it to fit more comfortably.
Supportive Devices for Symptom Management
There are a wide variety of devices and treatments that can be used to treat many of the coexisting symptoms that heart failure patients may be experiencing. These symptoms may be because of the disease itself or may be related to comorbidities or independently occurring problems.
CPAP / BiPAP / APAP
Continuous positive airway pressure (CPAP), bi-level positive airway pressure (BiPAP), or automatic positive airway pressure (APAP) are commonly used to treat sleep apnea. Sleep apnea is a condition in which a person ceases respirations while asleep for a period of 10 seconds or longer, sometimes gasping for air when respirations resume. The most common cause is obesity. This can result in hypertension, hypoxia, and tachycardia during the period of breathlessness, resulting in a CVA or MI occurring during sleep.
A CPAP, BiPAP, or APAP device is connected to a face mask or nasal pillow to deliver positive pressure to force the airway to remain open and allow the passage of air. The differences among these devices include: CPAP delivers a continuous pressure, BiPAP delivers different pressures for inhalation and exhalation, and APAP delivers pressure within a set range only when the person experiences a period of apnea. If the patient continues to be hypoxic during the cessation of apnea, oxygen can be spliced into the PAP tubing to increase the amount of oxygen available to the patient (Harding et al., 2020).
CARDIAC RESYNCHRONIZATION THERAPY
Cardiac resynchronization therapy (CRT) is a well-established method for improving cardiac function and coordinating biventricular contractions. CRT improves left ventricular function and therefore hemodynamic efficiency or cardiac output. A small titanium device containing a computer is surgically implanted into the chest wall and connects to wires extending to each ventricle. It acts similarly to a cardiac pacemaker but causes the ventricles to contract in time with each other. In patients for whom these therapies are successful, the outcomes are relief of HF symptoms, improved exercise function, and increased life expectancy.
Biventricular pacing improves ventricular function by resynchronizing the heartbeat through pacing both ventricles. This is used for treating HF patients with intraventricular conduction delays, which cause the right and left ventricles to beat out of synch with each other, leading to decreased systolic function, an inefficient pump, and aggravated HF (Harding et al., 2020).
Human Stem Cell Therapy
Embryonic stem cells (ESC) that have been altered to become cardiopoietic cells (cardiac-committed) show promise of reversing necrosis in cardiac tissue. When differentiated ESC are amplified to become cardiac progenitor cells, they are imbedded in a fibrin patch into the damaged cardiac tissue. This improves cardiac function and reduces the severity of heart failure by restoring the ability to pump to some previously akinetic (unmoving) areas of the myocardium. In one study, the ejection fraction improved from 38% to 45% ±3%, on average (Yamada et al., 2020).
Stem cells may also be derived from an individual’s own bone marrow or from their own healthy cardiac tissue, bringing about an improvement in their cardiac tissue. However, after approximately one week of cardiac muscle damage, the heart stops signaling for help in the form of stem cells, and the damage repair remains largely incomplete. More recent studies have shown that the introduction of highly selected donor stem cells, the individual’s own healthy cardiac tissue given at the time of a myocardial infarction (MI), or the individual’s own healthy cardiac tissue or donor cardiac tissue given late after an MI, to be more effective for tissue repair (Cleveland Clinic, 2021).
Cardiac Monitoring
Cardiac monitoring devices—particularly ambulatory devices such as wearable devices, smartphones, and other sensors—have become popular for continual monitoring of cardiac diseases such as HF. Such devices provide early detection of physiological events and can alert patients and clinicians of the need to seek medical attention.
Wearable monitoring devices and smartphone-based solutions can provide continuous cardiac monitoring and ambulatory monitoring capabilities for:
- ECG
- Heart rate
- Arrhythmia
- Blood pressure
- Cardio-respiratory fitness
- Stress
- Respiratory rate
- Temperature
- Oxygen saturation
- Ischemia
- Apnea
(Sana et al., 2020)
Implanted devices include the FDA-approved CardioMEMS HF system, which is implanted in the pulmonary artery during a right heart catheterization to measure pulmonary pressures. It is used on HF patients with NYHA Class III heart failure symptoms. HF treatment such as drug dosages are then adjusted based on the pulmonary artery pressure (PAP) reading. The patient-initiated readings are wirelessly transmitted to a secure website and monitored by the patient’s physicians (Harding et al., 2020).
Nutritional Therapy
Nutritional therapy is essential in treating an individual with heart failure. A nutritional consultation with a registered dietitian is usually performed during the initial hospital visit for HF during phase 1 of cardiac rehabilitation (see also “Cardiac Rehabilitation” below). This consultation is usually ordered to provide the patient and close family members with information to control the exacerbation or intensification of HF symptoms. Failure to comply with a low-sodium, fluid-restricted diet is one of the most likely reasons for a patient with HF to be readmitted to the hospital.
WEIGHT REDUCTION
Weight reduction is a key component in treating HF and preventing a worsening of symptoms. Obesity contributes to hypertension, which increases systemic vascular resistance and causes the ventricles to work harder to pump blood. Many HF patients, with or without hypertension, are advised to start a DASH (Dietary Approaches to Stop Hypertension) diet. This diet supports weight loss through consumption of fruits and vegetables, low- or nonfat dairy, lean meats, beans, seeds, nuts, and less sugar (Harding et al., 2020).
FLUID RESTRICTION
Fluid restriction is a treatment modality used to prevent volume overload, which can be caused by the accumulation of fluid in the peripheral tissues (resulting in edema in right-sided HF) or in the lungs (resulting in pulmonary edema in left-sided HF).
Fluid restriction is advised for patients with moderate or severe HF, especially those with renal disease as a comorbidity. Liquids from all sources are usually limited to less than 2,000 ml per day or per healthcare provider orders. HF patients are encouraged to weigh themselves daily at the same time of day and in similar clothing to monitor sudden weight gains indicative of fluid retention.
When the heart is unable to pump fluid effectively, a lower circulating volume reduces the cardiac workload and decreases the possibility of fluid retention and edema. In the hospital, it is the nurse’s responsibility to maintain a strict intake and output measurement when a patient has a fluid restriction ordered. The nurse records and reports the intake and output (I & O) fluid balance each shift and every 24 hours to ensure the patient is not retaining fluid. Nursing observations of jugular vein distension, peripheral edema, and lung sounds are also essential.
SODIUM RESTRICTION
Patients with heart failure are usually counseled to restrict sodium intake (1–2 grams/day) to prevent fluid retention. The affinity that sodium and water have for each other suggests that limiting the intake of sodium will prevent the kidneys from retaining fluid due to the increased serum sodium levels. The retained fluid can then lead to peripheral and dependent edema in earlier stages in HF and hypertension or shortness of breath in later stages.
Studies about the reduction of HF that relate directly to restricting sodium intake are inconclusive. Sodium restriction is usually recommended as an adjunct for HF patients for the possible benefits but is not considered to be effective without the concurrent use of pharmaceutical agents.
This is an area of some controversy, with conflicting study results indicating that the practice of sodium restriction cannot be considered evidence-based. A search of the literature does not provide clear proof of a threshold level of acceptable sodium intake for a HF patient. Historical recommendations have been for a moderate restriction of 2,000 mg of sodium per day for a person with HF with mild to moderate fluid retention and 1,000 mg per day for those with severe fluid retention. Recent studies, however, do not support these recommendations (Sole et al., 2020).
AHA HEART FAILURE GUIDELINES
Since 2016, the American Heart Association has provided hospitals with evidenced-based guidelines to promote adherence to proven protocols for HF patients designed to shorten hospital stays, reduce hospital readmissions, and maximize reimbursement for hospitals from Medicare, Medicaid, and private insurers. The guidelines upon discharge include:
- Prescription of angiotensin-converting enzyme (ACE) inhibitor, angiotensin reception blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNi), if appropriate for that patient
- Prescription of a beta-adrenergic blocking agent (beta-blocker), if appropriate
- Prescription of a combination of hydralazine and isosorbide dinitrate for African American patients, if appropriate
- Prescription of an aldosterone agonist, if appropriate
- Assessment of left ventricular function
- Establishment of a postdischarge appointment within 7 days of discharge
- Deep vein thrombosis (DVT) prevention measures for nonambulatory patients
- 60 minutes of HF education by a qualified heart failure educator
- Maintenance of BP <140 systolic and <90 diastolic
- A written copy of dietary instructions
- A written copy of discharge instructions
- A follow-up phone call within 48 to 72 hours of discharge
(Yancy et al., 2017)