OSHA BLOODBORNE PATHOGENS STANDARD
The Occupational Safety and Health Administration, part of the U.S. Department of Labor, first published the Occupational Exposure to Bloodborne Pathogens Standard in 1991 in Title 29 of the Code of Federal Regulations 1910.1030. In 2001, in response to the Needlestick Safety and Prevention Act, OSHA revised the Bloodborne Pathogens Standard.
The Bloodborne Pathogens Standard continues to be updated regularly, with the most recent update from April 2012 (see “Resources” at the end of this course). The Standard details what employers must do to protect workers whose jobs put them at risk for exposure to blood and other potentially infectious materials. OSHA regularly inspects healthcare agencies for compliance and may fine employers if infractions are identified.
BLOOD AND OTHER POTENTIALLY INFECTIOUS MATERIALS
All occupational exposures to blood or other potentially infectious materials place workers at risk for infection with bloodborne pathogens.
OSHA defines blood as:
- Human blood
- Human blood components
- Products made from human blood
Other potentially infectious materials (OPIM) include:
- Semen
- Vaginal secretions
- Cerebrospinal fluid
- Synovial fluid
- Pleural fluid
- Pericardial fluid
- Peritoneal fluid
- Amniotic fluid
- Saliva in dental procedures
- Any body fluid that is visibly contaminated with blood
- All body fluids in situations where it is difficult or impossible to differentiate between body fluids
- Any unfixed tissue or organ (other than intact skin) from a human (living or dead)
- HBV- and HIV-containing cell or tissue cultures, organ cultures, and HBV- or HIV-containing culture medium or other solutions
- Blood, organs, or other tissues from experimental animals infected with HBV or HIV
- Human breast milk (implicated in transmitting HIV and HBV from mother to infant and being an exposure risk for healthcare workers frequently exposed to breast milk)
(OSHA, 2012; CDC, 2020; CDC, 2015)
In general, OSHA’s Bloodborne Pathogens Standard (OSHA, 2012) requires employers to do the following:
- Establish a written exposure control plan designed to eliminate or minimize employee exposure to bloodborne pathogens. Employers must:
- Prepare an exposure determination that contains a list of job classifications in which all workers have occupational exposure and a list of job classifications in which some workers have occupational exposure, along with a list of the tasks and procedures performed by those workers that could result in exposure
- Ensure that a copy of the exposure control plan is accessible to employees
- Update the exposure control plan at least annually to reflect changes in tasks, procedures, and positions that affect occupational exposure, and also technological changes implemented to eliminate or reduce occupational exposure. Employers must:
- Annually document in the plan that they have considered and begun using appropriate, commercially available, and effective safer medical devices designed to eliminate or minimize occupational exposure
- Document that they have solicited input from frontline workers in identifying, evaluating, and selecting effective engineering and work practice controls
- Implement the use of Universal Precautions
- Universal Precautions means treating all human blood and other potentially infectious materials as if known to be infectious for bloodborne pathogens.
- Under circumstances in which differentiation between body fluid types is difficult or impossible, all body fluids shall be considered potentially infectious materials.
- Identify and use engineering controls
- These are devices that isolate or remove the bloodborne pathogens hazard from the workplace. They include sharps disposal containers, self-sheathing needles, and safer medical devices, such as sharps with engineered sharps-injury protection and needleless systems.
- Engineering controls shall be examined and maintained or replaced on a regular schedule to ensure their effectiveness.
- Identify and ensure the use of work practice controls
- These are practices that reduce the possibility of exposure by changing the way a task is performed, such as appropriate practices for handling and disposing of contaminated sharps, handling specimens, handling laundry, and cleaning contaminated surfaces and items.
- Employers shall provide handwashing facilities that are readily accessible to employees. When this is not feasible, appropriate antiseptic hand cleanser in conjunction with clean cloth/paper towels or antiseptic towelettes shall be available.
- Employers should ensure that employees wash their hands immediately or as soon as feasible after removal of gloves or other personal protective equipment.
- Use labels and signs to communicate hazards
- Warning labels must be affixed to containers of regulated waste; containers of contaminated reusable sharps; refrigerators and freezers containing blood or OPIM; other containers used to store, transport, or ship blood or OPIM; contaminated equipment that is being shipped or serviced; and bags or containers of contaminated laundry.
- Facilities may use red bags or red containers instead of labels.
- In HIV and HBV research laboratories and production facilities, signs must be posted at all access doors when OPIM or infected animals are present in the work area or containment module.
- Provide personal protective equipment (PPE), such as, but not limited to, gloves, gowns, laboratory coats, face shields or masks and eye protection, mouthpieces, resuscitation bags, pocket masks, or other ventilation devices
- Employers must clean, repair, and replace this equipment as needed. Provision, maintenance, repair, and replacement are at no cost to the worker.
- Make available hepatitis B vaccinations to all workers with occupational exposure
- Vaccination must be offered after the worker has received the required bloodborne pathogens training and within 10 days of initial assignment to a job with occupational exposure.
- Make available postexposure evaluation and follow-up to any occupationally exposed worker after an exposure incident
- An exposure incident is a specific eye, mouth, other mucous membrane, nonintact skin, or parenteral contact with blood or OPIM.
- Evaluation and follow-up must be at no cost to the worker and includes documenting the route(s) of exposure and the circumstance under which the exposure incident occurred; identifying and testing the source individual for HBV and HIV infectivity if the source individual consents or the law does not require consent; collecting and testing the exposed worker’s blood, if the worker consents; offering postexposure prophylaxis; offering counseling; and evaluating reported illnesses.
- The healthcare professional will provide a limited written opinion to the employer, and all diagnoses must remain confidential.
- Provide information and training to employees that covers all elements of the Standard, including, but not limited to, information on bloodborne pathogens and diseases, methods used to control occupational exposure, hepatitis B vaccine, and medical evaluation and postexposure follow-up procedures.
- Employers must offer this training on initial assignment, at least annually thereafter, and when new or modified tasks or procedures affect a worker’s occupational exposure.
- HIV and HBV laboratory and production facility workers must receive specialized initial training in addition to the training provided to all workers with occupational exposure. Workers must have the opportunity to ask the trainer questions. Training must be presented at an educational level and in a language that workers understand.
- Maintain employee training and medical records, including a sharps injury log
ENGINEERING CONTROL DEVICE EXAMPLES
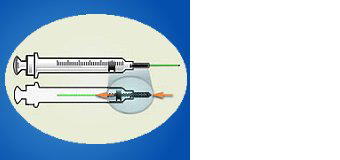
Syringe with retractable needle.
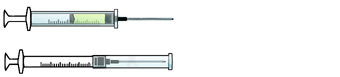
Self-resheathing needle.

Resheathing disposable scalpel.

Phlebotomy needle with hinged shield as an add-on safety feature.
(OSHA, 2020)
WARNING LABELS
Warning labels are fluorescent orange, red, or orange-red. Bags used to dispose of regulated waste must be red or orange-red, and they too must have the biohazard symbol in a contrasting color readily visible upon them (OSHA, 2012).

Biohazard warning label. (Source: OSHA.)