CAUSES OF TYPE 2 DIABETES
The etiology of type 2 diabetes is believed to be the result of complex interactions between environmental and genetic factors. The disease develops in response to a diabetes-prone lifestyle (i.e., excessive caloric intake, obesity, lack of exercise) in conjunction with a susceptible genotype.
Genetic Causes
Some aspects of all these predisposing problems are inherited, and in this way, the propensity for developing type 2 diabetes is inherited. The specific genetic causes are not known in detail for most variants of type 2 diabetes, but most cases appear to be polygenic—that is, they involve more than one inherited problem (ADA, 2020b).
The genetics of type 2 diabetes are not completely known. They are complex, and current evidence suggests that multiple genes in pancreatic beta cell failure and insulin resistance are involved. Specifically identified genetic variants account for about 10% of the heritable component of most cases of type 2 diabetes.
Some forms of diabetes have an evident link to genetic abnormalities. The syndrome historically known as maturity onset diabetes of youth (MODY) is now known be a variety of defects in beta cell function. This accounts for 2% to 5% of persons with type 2 diabetes who present at a young age and have only mild disease (ADA, 2020b).
Diabetes can also be found in other, more severe mitochondrial disorders such as Kearns-Sayre syndrome and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episode (MELAS). Some research also suggests that a genetically associated low birth weight increases a person’s risks for developing type 2 diabetes (Khardori, 2020).
Insulin Resistance
Insulin resistance is a molecular problem in which most tissues do not respond normally to insulin in the bloodstream, whether the insulin has been secreted by the pancreas or has been administered therapeutically.
Insulin resistance is the predominant factor that leads to type 2 diabetes, gestational diabetes, and prediabetes. When the body becomes resistant to insulin, it attempts to compensate by producing more insulin. Thus, individuals with insulin resistance are frequently producing more insulin than those who are healthy. Producing too much insulin is referred to as hyperinsulinemia.
Some research shows that insulin resistance can be reduced by following low-carbohydrate and ketogenic diets (Diabetes.co.uk, 2017). Ketogenic diets are high-fat, adequate protein, and low-carbohydrate. This type of diet alters the way energy is used in the body. Fat is converted into fatty acids and ketone bodies. This helps to lower glucose levels and reduces insulin resistance. Others caution the use of a ketogenic diet due to the high fat content (especially unsaturated fats) combined with eating fewer nutrient-rich fruits and vegetables for long-term cardiovascular health (Abasi, 2018).
EFFECTS OF INSULIN RESISTANCE
In a person with insulin resistance, a normal amount of circulating insulin produces:
- Less than the normal amount of glucose transport into cells
- Less than the normal use of intracellular glucose
- Less than the normal storage of glucose in the form of glycogen
- More than the normal release of glucose into the circulation by the liver
All people with type 2 diabetes have insulin resistance. Insulin resistance exists in a person years before the diabetes is diagnosed, and the presence of insulin resistance in an asymptomatic person predicts the high probability of developing type 2 diabetes. Although diabetes is often thought of as a disease of the pancreas, insulin resistance is a problem in the cells throughout the body that respond to insulin. Usually, it is a problem in the molecular mechanisms by which cells recognize the insulin molecule and then produce the intracellular effects of this recognition.
There are many separate molecular sites that can be the source of insulin resistance. Insulin receptors (which are in the membranes of responding cells) are complex structures made of a number of separate subunits. The malfunctioning or mutation of any of these subunits can make them work inefficiently or make them insensitive to insulin, leading to insulin resistance. Insulin resistance can also be caused by the malfunctioning of any of the components of the intracellular cascade that connects the insulin receptors in the cell membrane to the glucose-processing machinery inside the cell.
EXCESS VISCERAL FAT
Intra-abdominal fat is strongly associated with insulin resistance—more so than is extra-abdominal (subcutaneous) fat. Intra-abdominal fat is visceral fat, and an overabundance of visceral fat cells both triggers and worsens insulin resistance.
About 90% of body fat is subcutaneous fat, which is the kind of fat that is felt when the skin is pinched. The remaining 10% is intra-abdominal fat, which is located beneath the abdominal muscles and can only be detected by MRI (Merck Manual, 2020a).
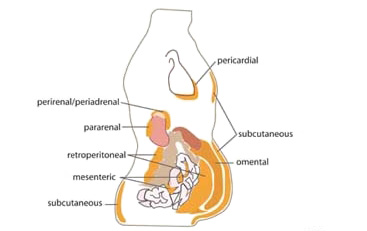
Abdominal fat distribution in the body, showing subcutaneous and various types of visceral fat. (Source: Cook A & Cowan C, Adipose (March 31, 2009), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.40.1, via Wikimedia Commons.)
Signals within the sympathetic nervous system cause fat cells to break down and release their stored fat. Insulin gives the opposite message: insulin signals fat cells to slow or stop the release of fat. Since visceral fat cells are less responsive to insulin, having too many visceral fat cells leads to too much free fatty acid in the bloodstream, and the high level of free fatty acid eventually leads to hyperglycemia.
Hyperglycemia stimulates the pancreas to release more insulin. In this way, the excess free fatty acids have indirectly triggered, at least temporarily, higher-than-normal levels of circulating insulin (i.e., hyperinsulinemia).
If it had been subcutaneous fat cells that were releasing the excess fatty acids, the newly released insulin would turn off the tap by slowing or stopping the fatty acid release. Visceral fat cells, however, are less sensitive to insulin signals, and the feedback circuit is not very effective. When visceral fat is the source of excess free fatty acids, the natural balancing mechanisms do not work well, and the hyperinsulinemia persists. This persistent hyperinsulinemia is a direct cause of insulin resistance.
FROM EXCESS FATTY ACIDS TO INSULIN RESISTANCE
- Persistent elevation of circulating free fatty acids causes hyperglycemia.
- Persistent hyperglycemia causes hyperinsulinemia.
- Persistent hyperinsulinemia causes insulin resistance.
This sequence of events shown in the box above can be expressed as the formula:
Fatty acids → Hyperglycemia → Hyperinsulinemia → Insulin resistance
The sequence can be triggered by anything that causes high blood levels of free fatty acids, glucose, or insulin. Conditions that lead to insulin resistance through this mechanism include high levels of glucocorticoids (e.g., Cushing’s disease or long-term treatment with prednisone), nonalcoholic fatty liver disease, and chronic elevated triglyceride levels.
OBESITY
Obesity has long been associated with a risk for type 2 diabetes. Risk factors for obesity include:
- Genetics. Genes may affect the amount of body fat a person has and where it is distributed. Genetics may also influence how efficiently the body converts food into energy and how the body burns calories during exercise.
- Family lifestyle. Family members generally share similar eating and activity behaviors.
- Inactivity. Without adequate exercise, people take in more calories than they burn, which can lead to weight gain.
- Unhealthy diet. Unhealthy diets can easily lead to obesity. Such diets are generally high in calories; lack adequate amounts of fruits and vegetables; and include fast food, oversized portions, and high-calorie beverages.
- Medical conditions. Cushing’s syndrome and/or conditions that decrease activity, such as arthritis, can lead to weight gain.
- Medications. Some medications that can lead to weight gain include certain classes of antidepressants, antiseizure medications, and beta blockers.
- Age. As one ages, hormonal changes and a less active lifestyle can contribute to weight gain.
- Lack of sleep. Getting too much or too little sleep can cause hormonal changes that increase appetite.
(Merck Manual, 2020a)
Because obesity puts a person at risk for type 2 diabetes, all the causes of obesity, from genes to lifestyle habits to medications, can contribute to a person’s tendency to develop type 2 diabetes (ADA, 2020a; Merck Manual, 2020a).
DRUGS THAT CAN CAUSE WEIGHT GAIN
- Psychiatric drugs (e.g., lithium, antipsychotics such as chlorpromazine and clozapine, antidepressants such as tricyclics)
- Neurologic drugs (e.g., anti-epileptic drugs such as valproate)
- Steroids (e.g., hormonal contraceptives, prednisone)
- Antidiabetic drugs (e.g., insulin, sulfonylureas)
- Antihistamines
- Beta blockers
(Comerford, 2017)
IMMUNE SYSTEM ABNORMALITIES
There is now significant evidence to indicate that an overactive immune system response may actually target the beta cells of the pancreas, thus damaging these insulin-producing cells and adversely affecting insulin production. This phenomenon occurs mainly in patients with insulin-dependent diabetes and may be an indication of an autoimmune cause of the disease.
Abnormal Insulin Secretion
In addition to insulin resistance, people with type 2 diabetes have another key disorder. The beta cells in their pancreases do not secrete insulin normally. Together, insulin resistance and poorly functioning beta cells lead to the continual hyperglycemia that characterizes type 2 diabetes.
Insulin resistance means that a higher-than-normal amount of insulin in the bloodstream is needed to keep the plasma glucose levels at a normal level (<100 mg/dL). To maintain healthy blood glucose levels, the pancreatic beta cells in a person with insulin resistance are forced to secrete more than the normal amount of insulin. Therefore, people with insulin resistance generally have hyperinsulinemia.
People with type 2 diabetes have insulin resistance; therefore, they often have hyperinsulinemia. But even when they have hyperinsulinemia, the blood insulin levels are not high enough to prevent hyperglycemia. In other words, even when secreting high levels of insulin, their pancreas does not keep up with the demand. Part of the problem is that people with type 2 diabetes have fewer beta cells than normal. In addition, the existing beta cells in patients with type 2 diabetes do not secrete insulin as quickly and in as large amounts as normal.
Even before type 2 diabetes develops, beta cell problems can be detected in glucose tolerance tests, which give abnormal test results in prediabetic individuals. As with insulin resistance, beta cell dysfunction precedes the development of overt hyperglycemia by many years.
In another parallel with insulin resistance, treating type 2 diabetes can improve the functioning of the beta cells, but it cannot bring beta cell functioning up to normal. At present, both insulin resistance and beta cell dysfunction can be improved but not cured (Merck Manual, 2019a).
Metabolic Syndrome
Metabolic syndrome is the name for a particular group of characteristics or health problems that are frequently found together. It is also sometimes called insulin resistance syndrome, or syndrome X.
Core problems of metabolic syndrome are obesity and insulin resistance. Three additional problems are high blood pressure, high blood levels of triglycerides, and low blood levels of high-density lipoprotein cholesterol (HDL). It is not clear whether metabolic syndrome causes type 2 diabetes, but it has been shown that having the syndrome increases a person’s chances of developing type 2 diabetes and cardiovascular disease (Merck Manual, 2020b).
DEFINITION OF METABOLIC SYNDROME
A diagnosis of metabolic syndrome is made if at least three of the following are present:
- Large waist circumference: a waistline that measures ≥35 inches (89 cm) for women and ≥40 inches (102 cm) for men
- Hypertriglyceridemia: ≥150 mg/dL or 1.7 millimoles per liter
- Low high-density lipoprotein (HDL) cholesterol: ≥40 mg/dL in men or ≥50 mg/dL in women of this “good” cholesterol
- High blood pressure: ≥130/85 mmHg
- High fasting glucose: ≥100 mg/dL
(Merck Manual, 2020b)
CASE
George is a 40-year-old male being treated for hypertension. He arrives to the clinic for an annual physical. After stepping onto a scale, he is found to have gained 10 pounds over the previous year. His blood pressure has gradually been increasing over the past two years as well, with a current measurement of 140/88.
As his medical and family history is taken, George mentions that his mother and uncle were both diagnosed with diabetes after age 50. The nurse takes a measurement of his waist circumference, which is 105 cm (41 in).
After discussing the clinical picture with the primary care physician, a lipid panel is ordered. Three days later, the results of George’s blood test show blood triglycerides of 156 mg/dL and an HDL cholesterol level of 38 mg/dL.
George is diagnosed with metabolic syndrome; he is started on an antilipemic agent and instructed on incorporating lifestyle interventions (e.g., diet, exercise) and given a referral to a dietitian at his request. A follow-up appointment is scheduled for three months later to assess how George is doing with initial management.
When George returns for his follow-up visit, he reports that he has been following his diet and exercise plan and feels that this has made a difference in how he is feeling. He has lost 8 pounds, his blood pressure is now 124/78, his triglycerides have improved to 130 mg/dL, and his HDL cholesterol has increased to 52 mg/dL.
George continues to be motivated to make changes in order to improve his health and states that he feels better than ever. He adds that his wife has been very supportive—together they are following a Mediterranean diet for meals and exercising on a regular basis.